Dimeric immune fusion protein, pharmaceutical composition and application
A fusion protein and dimer technology, applied in the direction of drug combination, fusion polypeptide, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1. Construction, expression and characterization of soluble dimer immune fusion protein
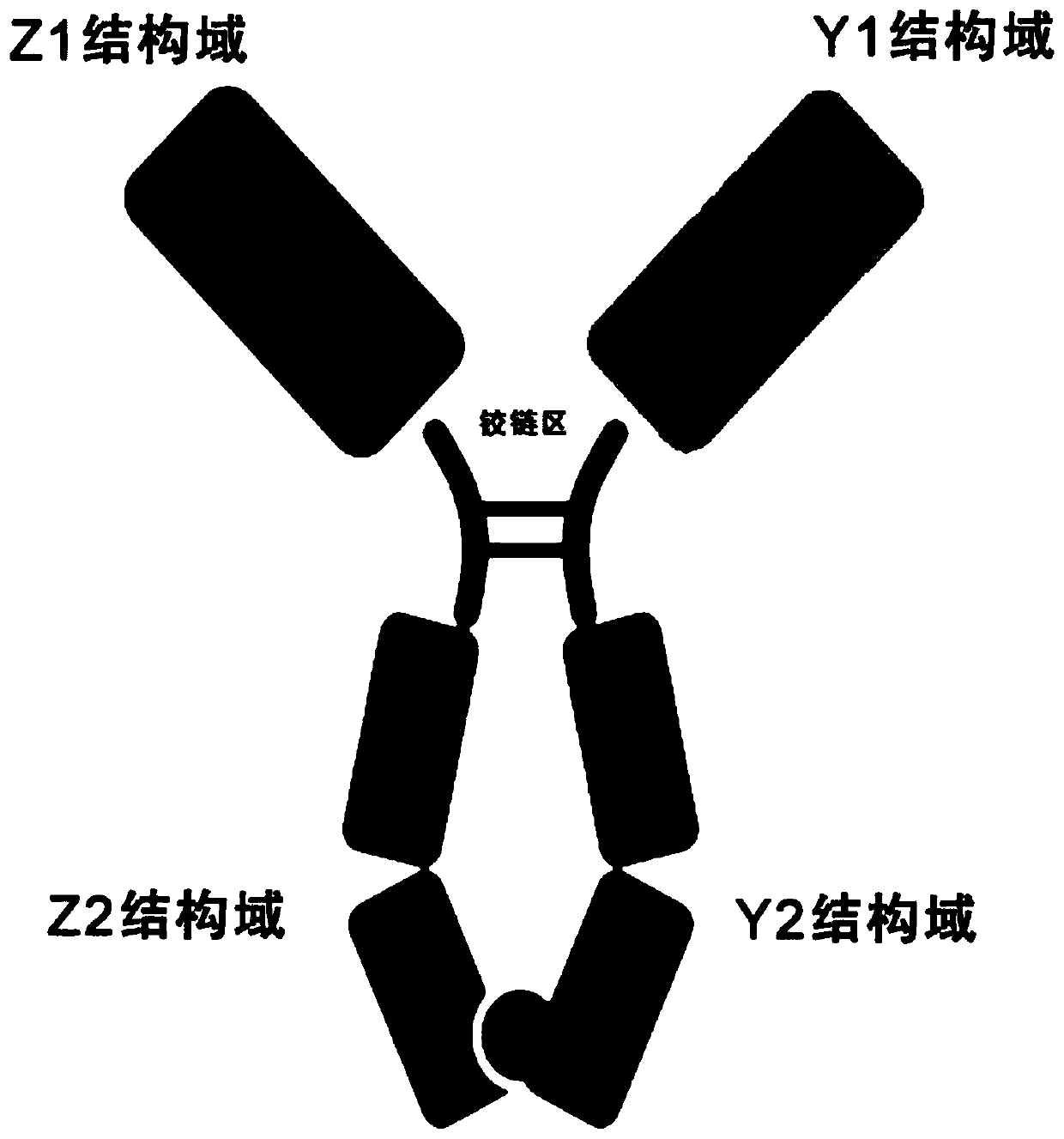
[0076] Such as figure 1 As shown, the soluble dimer immunofusion protein is a dimer with antibody Fc, and the method of constructing and expressing the dimer immunofusion protein itself is a routine experimental technique in the field, and a brief description is as follows:
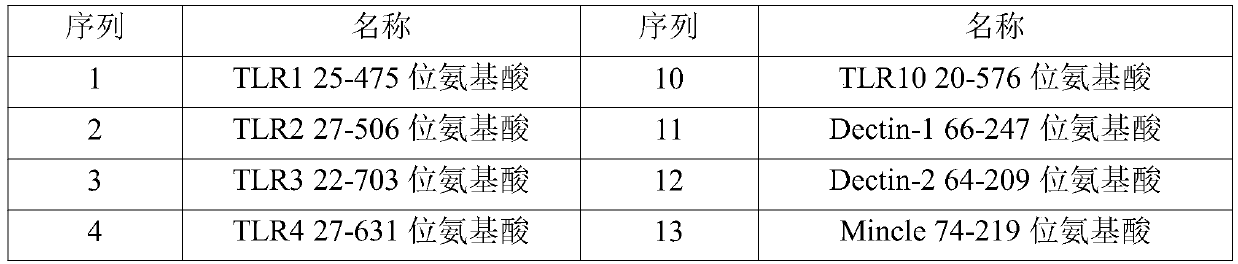
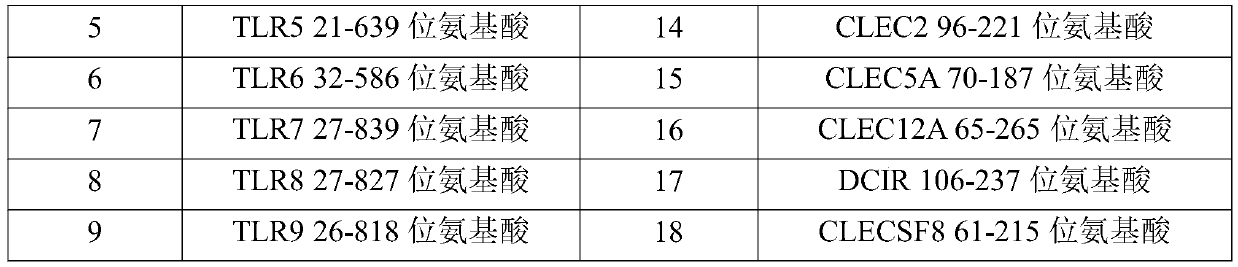
[0077] (1) Entrust a gene synthesizer (Suzhou Jinweizhi Company) to optimize the coding nucleotide codon and complete gene synthesis for the amino acid sequence of the dimer immune fusion protein required in this example, and the optimized nucleotide sequence is directly loaded into On the PCDNA3.4 vector, the amino acid sequences encoded by all vectors are described in Table 1.
[0078] (2) Entrust the protein manufacturer (Shenzhou Yiqiao Co., Ltd.) to perform expression and purification of the dimeric immunofusion protein for this example. Using literature Finck B K. Science, 265.; Mihara M et al...
Embodiment 2 2
[0088] Embodiment 2 dimer immune fusion protein is to Staphylococcus aureus effect
[0089] Staphylococcus aureus expressing green fluorescent protein was collected from the Institute of Microbiology, Chinese Academy of Sciences, and the multiplicity of infection was 1:10. Peripheral blood samples were collected from healthy volunteers to separate peripheral mononuclear cells (PBMC, separated by Biyuntian Lymphocyte Separation Kit). Freshly isolated cells were stabilized in RPMI1640 medium containing 10% fetal bovine serum for 2 h (37.5°C, 5% CO 2 ).
[0090] Cultivate Staphylococcus aureus according to the experimental requirements, collect the bacteria by centrifuging at 10000g for 30 seconds in a desktop centrifuge, and resuspend to 10 8 Bacterial density around CFU / ml. Take the bacterial suspension for serial dilution plate counting to determine the exact bacterial density. After the PBMC cells replaced the medium, add 10 microliters of Staphylococcus aureus suspension...
Embodiment 3
[0098] Example 3 Immunodimer Effects on Methicillin-resistant Staphylococcus aureus
[0099] BALB / c mice, SPF grade, female, 6-8 weeks old, weighing 18-20 g, international standard strain MRSA-252, were purchased from American Tissue Culture Collection (ATCC). To establish a mouse model, inject 0.1 mL of the washed bacterial solution (the concentration of the bacterial solution is 1×10 9 CFU / mL), the mice in the blank group were injected with the same amount of sterile saline through the tail vein. The mice were then divided into a control group and a treatment group, with 10 mice in each group. The control group was given control IgG, and the treatment group was given a representative of the dimer immune fusion protein of the present invention at a dose of 10 mg / kg, administered intravenously. Inject once a day and observe continuously for 10 days. If any mice died or all mice were killed at the end of the last day of the experiment, the blood was plated immediately under a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com