Ion Chromatographic Detection Method of Residual Nacnbh3 in Polysaccharide Conjugate Vaccines
A technology combining vaccines and ion chromatography, applied in the field of ion chromatography-pulse amperometric determination of NaCNBH3, can solve the problems of complex conditions, long reaction time, low sensitivity, etc., and achieve the effect of simple method, convenient operation and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
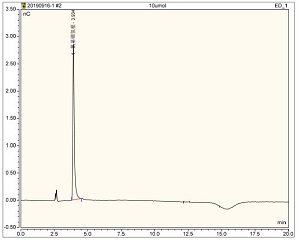
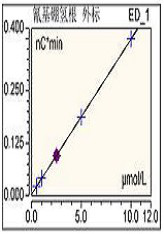
[0017] NaCNBH in Haemophilus influenzae type b conjugate vaccine stock solution 3 Residual determination:
[0018] 1. Precisely prepare 0.5μmol / L, 1.0μmol / L, 2.5μmol / L, 5μmol / L, 10μmol / L NaCNBH 3 standard solution;
[0019] 2. 30K for stock solution of Haemophilus influenzae type b conjugate vaccine D The ultrafiltration tube was centrifuged at 4000g for 20 minutes, and the filtrate was collected, diluted 10 times, and filtered with a 0.45 μm membrane as the test solution;
[0020] 3. The standard solution and the test solution are loaded and tested according to the chromatographic conditions;
[0021] Setting of chromatographic conditions:
[0022] 3.1 Mobile phase: 50mmol / L NaOH solution;
[0023] 3.2 Chromatographic column: AG11-HC, AS11-HC column, purchased from thermo company;
[0024] 3.3 Detector: pulsed amperometric detector, the reference electrode is silver chloride, and the detection waveform is "silver electrode, S-2, CN-";
[0025] 3.4 Detection method: chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com