High-content oxytetracycline injection and preparation method thereof
A technology of oxytetracycline and injection, applied in the field of veterinary high-content oxytetracycline injection and preparation thereof, can solve the problem of producing precipitation, optimal use ratio not mentioned, no glycerol formal and dimethylacetamide Use ratio studies etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
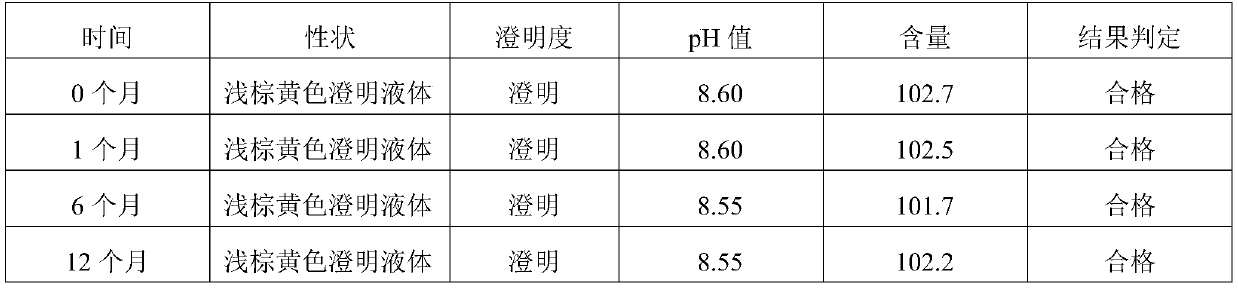
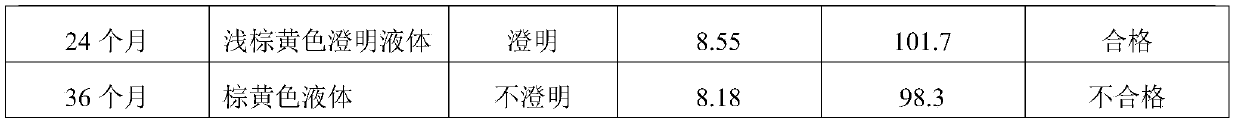
[0018] Example 1: Each 100ml of oxytetracycline injection contains 35g of oxytetracycline base, 2.4g of magnesium oxide, 1.0g of sodium formaldehyde sulfoxylate, 25g of glycerin formal, 40g of N,N-dimethylacetamide, monoethanolamine 1.6g, the rest is water for injection. (glycerol formal: dimethylacetamide is 2.5:4)
Embodiment 2
[0019] Example 2: Each 100ml of oxytetracycline injection contains 35g of oxytetracycline base, 2.4g of magnesium oxide, 1.0g of sodium formaldehyde sulfoxylate, 25g of glycerin formal, 35g of N,N-dimethylacetamide, monoethanolamine 1.6g, the rest is water for injection. (Glycerol formal: dimethylacetamide is 2.5:3.5)
Embodiment 3
[0020] Example 3: Each 100ml of oxytetracycline injection contains 35g of oxytetracycline base, 2.4g of magnesium oxide, 1.0g of sodium formaldehyde sulfoxylate, 25g of glycerin formal, 30g of N,N-dimethylacetamide, monoethanolamine 1.6g, the rest is water for injection. (glycerol formal: dimethylacetamide is 2.5:3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com