Method for converting arsenic residue into scorodite in one step
A technology of scorodite and transformation, applied in chemical instruments and methods, inorganic chemistry, iron compounds, etc., can solve the problems of polluting the atmospheric environment, explosion, complex process, etc., and achieve the effects of easy precipitation, large particles, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
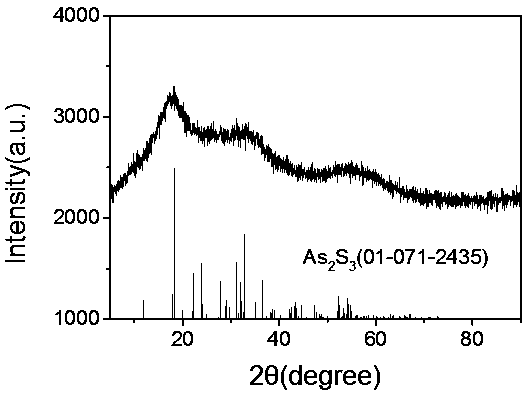
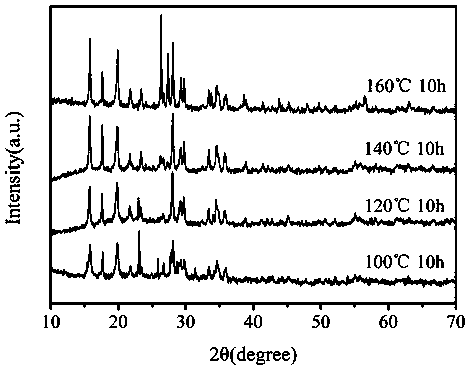
[0030] Take 0.1 g of arsenic sulfide slag in a reaction kettle, add 10 mL of 10wt% hydrogen peroxide, and 5 mL of 0.08 mol / L ferric sulfate solution, so that the solid-liquid ratio is 1:150 g / ml, and adjust the pH to 2 with sulfuric acid After stirring evenly, the reaction kettle was put into 100°C, 120°C, 140°C, and 160°C for 10 h, and cooled to room temperature naturally. Through cleaning and flotation, the yellow agglomerated sulfur element and the gray-green scorodite solid are finally separated, and the scorodite particle size increases with the increase of temperature, and the stability improves. Toxic leaching experiments showed that the toxic leaching of arsenic was less than 1.2 mg / L.
Embodiment 2
[0032] Take 0.1 g of arsenic sulfide slag in the reaction kettle, add 10 mL of 10wt% hydrogen peroxide, and 5 mL of 0.08~0.16 mol / L (Fe / As=1~2) ferric sulfate solution, so that the solid-liquid ratio is 1: 150 g / ml, using sulfuric acid to adjust pH = 2, after stirring evenly, put the reaction kettle at 160°C for 10 h, and let it cool down to room temperature naturally. Through cleaning and flotation, yellow agglomerated sulfur element and gray-green scorodite solid were finally separated. As the iron concentration increases, the arsenic fixation rate increases from 82% to 92%, and the particle size becomes smaller. When the molar ratio of iron to arsenic reaches 1.6:1, the product is a mixed phase of scorodite and iron oxide. The toxic leaching of arsenic in scorodite produced under these conditions was less than 1.5 mg / L.
Embodiment 3
[0034]Take 0.1 g of arsenic sulfide slag in a reaction kettle, add 10 mL of 10 wt% hydrogen peroxide, and 5 mL of 0.1 mol / L ferric sulfate solution, so that the solid-liquid ratio is 1:150 g / ml, and adjust the pH with sulfuric acid 1, 2 or 3. After stirring evenly, put the reaction kettle at 160°C for 10 h, and let it cool down to room temperature naturally. Through cleaning and flotation, yellow agglomerated sulfur element and gray-green scorodite solid were finally separated. The experimental results show that the arsenic in the arsenic sulfide slag can be fixed within the pH range of 1-3, and when the pH value is 2, the arsenic fixation rate can reach 91%, and the leaching toxicity concentration of arsenic is less than 1.0 mg / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com