Solid-state lithium ion composite electrolyte membrane and preparation method thereof
A composite electrolyte membrane and lithium-ion technology, which is applied in the field of solid-state lithium-ion composite electrolyte membrane and its preparation, can solve the problems of increased energy density of all-solid-state lithium-ion batteries, low room temperature ion conductivity, and small lithium ion migration number, etc., to achieve Easy to adsorb anions, enhance solubility, and uniform shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
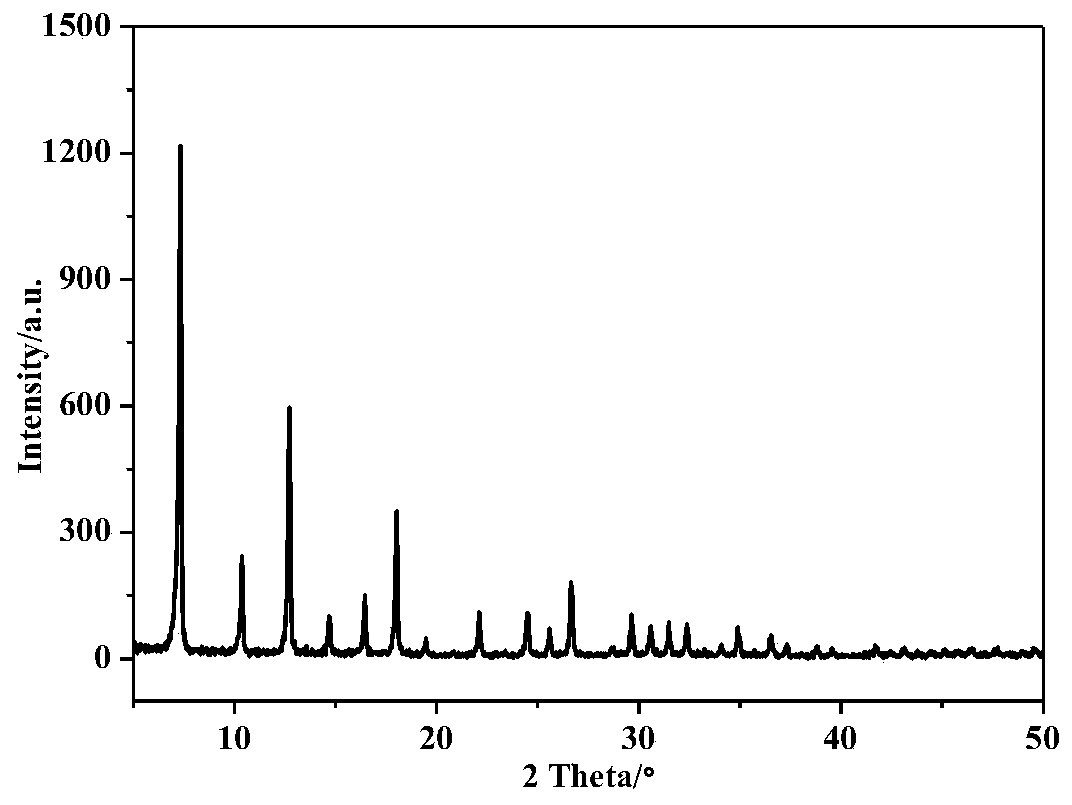
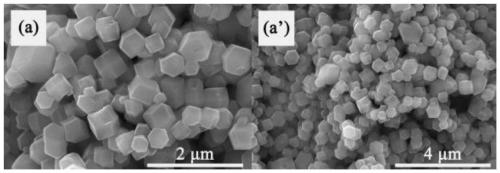
[0033] Take a 100mL clean beaker, add 25mL of methanol solvent into it, then weigh 1.4552g of cobalt nitrate and dissolve it in methanol solvent to obtain solution A; 2. Add 5133g of dimethylimidazole into a beaker filled with 25mL of methanol solvent, and magnetically stir to dissolve it completely to form solution B. Under the condition of magnetic stirring, add solution A to solution B drop by drop, let it stand for 24 hours after the dropwise addition, then centrifuge the formed precipitate at 8000 rpm for 3 minutes, and wash the precipitate with methanol for 3 times , the precipitate after centrifugation was transferred to a vacuum drying oven, and dried at a temperature of 60° C. for 12 hours to obtain an organometallic framework ZIF-67 material.
[0034] Weigh 0.6g of PEO and 0.2175g of LITFSI (the molar ratio of EO to LITFSI is 18:1), add them into 6mL of acetonitrile solvent respectively, stir them with magnetic force to make them completely dissolve, and then add 0....
Embodiment 2
[0036]Take a 100mL clean beaker, add 25mL of methanol solvent into it, then weigh 1.4552g of cobalt nitrate and dissolve it in methanol solvent to obtain solution A; 2. Add 5133g of dimethylimidazole into a beaker filled with 25mL of methanol solvent, and magnetically stir to dissolve it completely to form solution B. Under the condition of magnetic stirring, add solution A to solution B drop by drop, let it stand for 24 hours after the dropwise addition, then centrifuge the formed precipitate at 8000 rpm for 3 minutes, and wash the precipitate with methanol for 3 times , the precipitate after centrifugation was transferred to a vacuum drying oven, and dried at a temperature of 60° C. for 12 hours to obtain an organometallic framework ZIF-67 material.
[0037] Weigh 0.6g of PEO and 0.2175g of LITFSI (the molar ratio of EO to LITFSI is 18:1), add them into 6mL of acetonitrile solvent respectively, stir them with magnetic force to make them completely dissolve, and then add 0.0...
Embodiment 3
[0039] Take a 100mL clean beaker, add 25mL of methanol solvent into it, then weigh 1.4552g of cobalt nitrate and dissolve it in methanol solvent to obtain solution A; 2. Add 5133g of dimethylimidazole into a beaker filled with 25mL of methanol solvent, and magnetically stir to dissolve it completely to form solution B. Under the condition of magnetic stirring, add solution A to solution B drop by drop, let it stand for 24 hours after the dropwise addition, then centrifuge the formed precipitate at 8000 rpm for 3 minutes, and wash the precipitate with methanol for 3 times , the precipitate after centrifugation was transferred to a vacuum drying oven, and dried at a temperature of 60° C. for 12 hours to obtain an organometallic framework ZIF-67 material.
[0040] Weigh 0.6g of PEO and 0.2175g of LITFSI (the molar ratio of EO to LITFSI is 18:1), add them into 6mL of acetonitrile solvent respectively, stir them with magnetic force to make them completely dissolve, and then add 0....
PUM
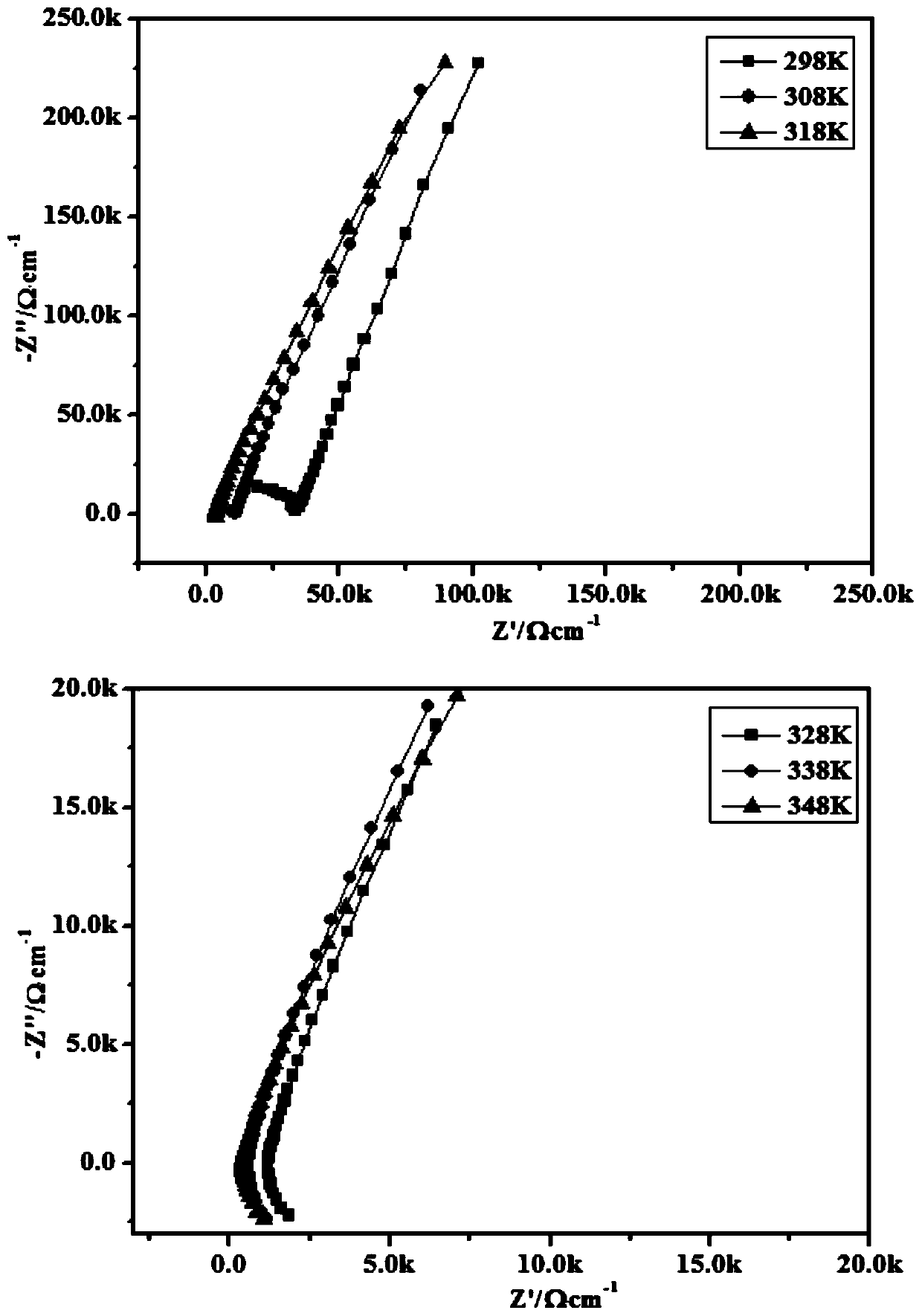
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com