Catalyst suitable for producing acrolein, and applications thereof
A catalyst, acrolein technology, applied in physical/chemical process catalysts, heterogeneous catalyst chemical elements, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as low yield of acrolein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
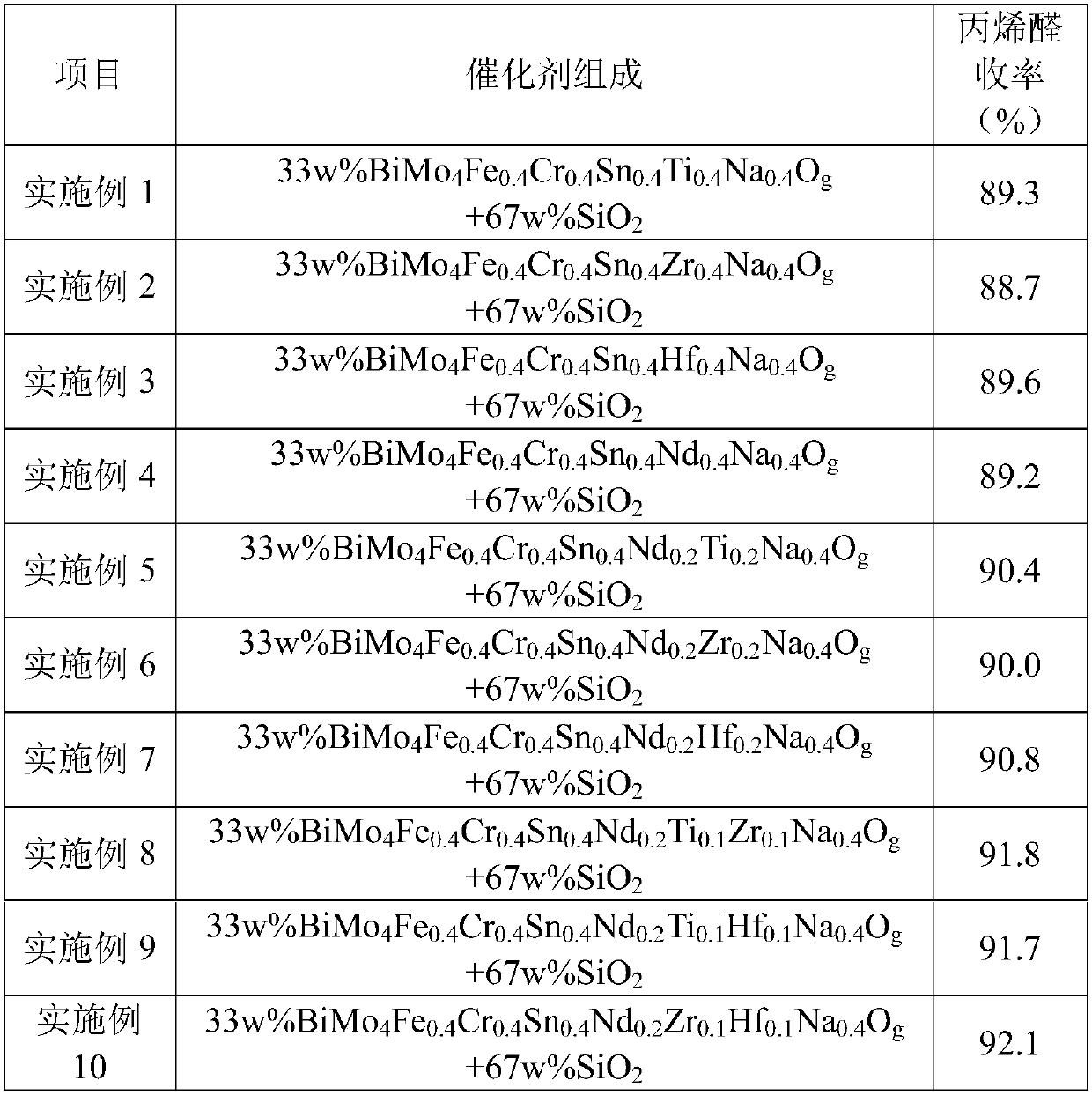
[0066] 1. Catalyst preparation
[0067] Bismuth nitrate (molecular formula is: Bi(NO 3 ) 3 ) was dissolved in 200g of hot water at 80°C. Ammonium molybdate (molecular formula is: (NH 4 ) 2 MoO 4 ) into it, and then respectively add chromium nitrate containing 0.04 moles of Cr (molecular formula: Cr(NO 3 ) 3 ), tin dioxide containing 0.04 moles of Sn (molecular formula: SnO 2 ), titanium dioxide containing 0.04 moles of Ti (molecular formula: TiO 2 ), containing 0.04 moles of Na sodium nitrate (molecular formula is NaNO 3 ), stirred to make it all dissolve to obtain solution I. Ferric nitrate containing 0.04 moles of Fe (molecular formula is: Fe(NO 3 ) 3 ) aqueous solution was added to the above solution, mixed evenly, and evaporated at 80°C until the mixture containing the active component BiMo 4 Fe 0.4 Cr 0.4 sn 0.4 Ti 0.4 Na 0.4 o g The concentration of 0.4g / g, to obtain solution II.
[0068] Mix 200 g of spherical silica carrier with a diameter of 5 mm an...
Embodiment 2
[0079] 1. Catalyst preparation
[0080] Bismuth nitrate (molecular formula is: Bi(NO 3 ) 3 ) was dissolved in 200g of hot water at 80°C. Ammonium molybdate (molecular formula is: (NH 4 ) 2 MoO 4 ) into it, and then respectively add chromium nitrate containing 0.04 moles of Cr (molecular formula: Cr(NO 3 ) 3 ), tin dioxide containing 0.04 moles of Sn (molecular formula: SnO 2 ), zirconium nitrate (molecular formula: Zr(NO 3 ) 4 ), containing 0.04 moles of Na sodium nitrate (molecular formula is NaNO 3 ), stirred to make it all dissolve to obtain solution I. Ferric nitrate containing 0.04 moles of Fe (molecular formula is: Fe(NO 3 ) 3 ) aqueous solution was added to the above solution, mixed evenly, and evaporated at 80°C until the mixture containing the active component BiMo 4 Fe 0.4 Cr 0.4 sn 0.4 Zr 0.4 Na 0.4 o g The concentration of 0.4g / g, to obtain solution II.
[0081] Mix 200 g of spherical silica carrier with a diameter of 5 mm and 200 g of solution ...
Embodiment 3
[0092] 1. Catalyst preparation
[0093] Bismuth nitrate (molecular formula is: Bi(NO 3 ) 3 ) was dissolved in 200g of hot water at 80°C. Ammonium molybdate (molecular formula is: (NH 4 ) 2 MoO4 ) into it, and then respectively add chromium nitrate containing 0.04 moles of Cr (molecular formula: Cr(NO 3 ) 3 ), tin dioxide containing 0.04 moles of Sn (molecular formula: SnO 2 ), hafnium oxide containing 0.04 moles of Hf (molecular formula: HfO 2 ), containing 0.04 moles of Na sodium nitrate (molecular formula is NaNO 3 ), stirred to make it all dissolve to obtain solution I. Ferric nitrate containing 0.04 moles of Fe (molecular formula is: Fe(NO 3 ) 3 ) aqueous solution was added to the above solution, mixed evenly, and evaporated at 80°C until the mixture containing the active component BiMo 4 Fe 0.4 Cr 0.4 sn 0.4 Hf 0.4 Na 0.4 o g The concentration of 0.4g / g, to obtain solution II.
[0094] Mix 200 g of spherical silica carrier with a diameter of 5 mm and 200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com