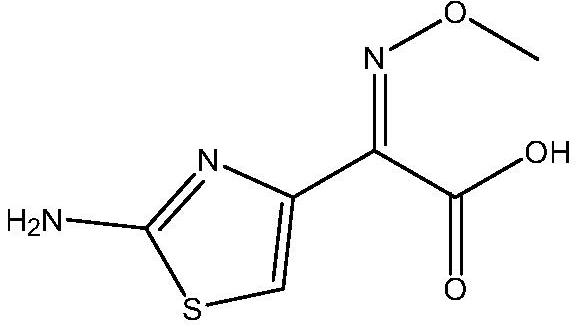
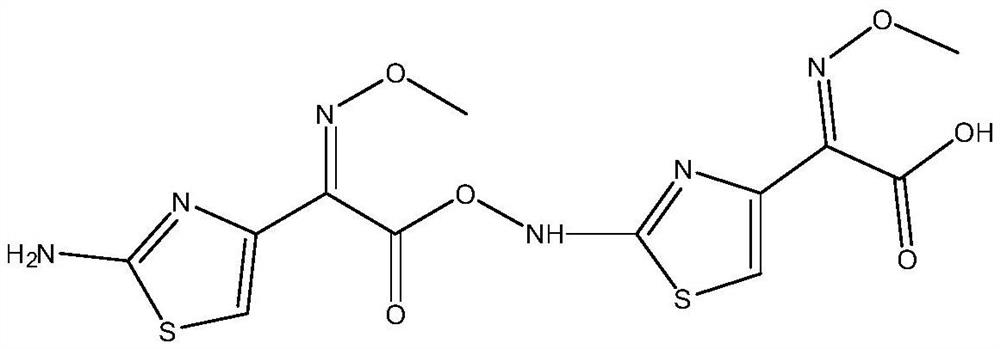
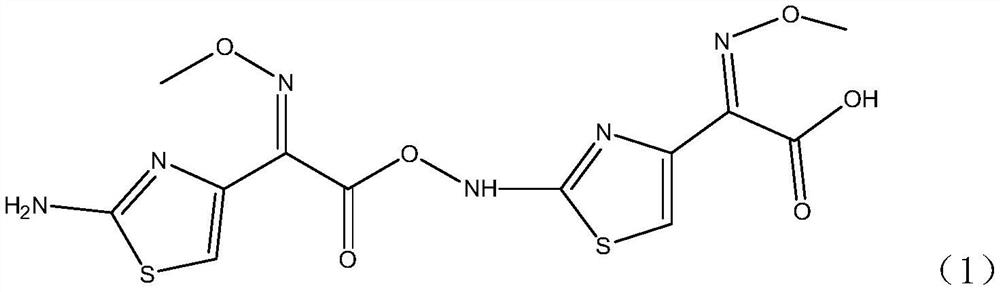
A high-purity selective preparation and purification method of aminothioxamic acid dimer
A technology of aminothiaxamic acid dimer and purification method, which is applied in the direction of organic chemistry, can solve the problem of no dimer being found, achieve the effect of improving product purity, simplifying the process flow, and realizing selective preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 100ml of dichloromethane into a 250ml four-necked bottle, add 20.8g of athioxamic acid, cool down to 0°C, add 8.4g of diethylamine, control the temperature at 0-2°C, and stir for 60 minutes until it dissolves. In another 500ml four-neck bottle, add 150ml of dichloromethane, 21g of athioxamic acid, cool down to 0°C, add 11g of triethylamine, control the temperature at 0-2°C, stir for 60min until dissolved, cool down to -35°C, add 13.2g of pivaloyl chloride , N-methylmorpholine 0.83g, react at -20°C for 60min under temperature control.
[0042] Add the solution to the mixed anhydride solution, add 1g of DCC, control the temperature at -20°C, add triethylamine dropwise during the process to control the pH=7.5, and react for 5 hours to obtain a light yellow suspension. Suction filtration, the filtrate is at 30°C, -0.08Mpa Distill under vacuum until no distillate flows out. Add 100ml of water dropwise over 1.5h to precipitate a light yellow solid, filter and wash the fil...
Embodiment 2
[0044] Add 80ml of acetonitrile to a 250ml four-necked flask, add 20.8g of acetioxamic acid, cool down to 0°C, add 9.4g of tetramethylguanidine, control the temperature at 5°C, and stir for 60 minutes until it dissolves. Another 500ml four-neck bottle was added with 180ml of acetonitrile, 21g of aminothioxamic acid, cooled to 0°C, added 10g of diethylamine, controlled temperature at 5°C, stirred for 60min until dissolved, cooled to -30°C, added 13.2g of pivaloyl chloride, N-formazol 0.83 g of morpholine was reacted at -15°C for 60 minutes.
[0045]Add the solution to the mixed anhydride solution, add 1.5g of BSA, control the temperature at -15°C, add diethylamine dropwise during the process to control the pH=8.0, and react for 3 hours to obtain a light yellow suspension. Suction filtration, the filtrate is at 50°C, - Distill under vacuum condition of 0.08Mpa until no distillate flows out, add 200ml of water dropwise in 2 hours, precipitate a light yellow solid, filter, wash th...
Embodiment 3
[0047] Add 120ml of ethyl acetate to a 250ml four-necked bottle, add 20.8g of aminothiaxamic acid, cool down to 0°C, add 7.8g of triethylamine, control the temperature at 5°C, and stir for 60 minutes until dissolved. In another 500ml four-necked bottle, add 180ml of ethyl acetate, 21g of aminothiaxamic acid and cool down to 0°C, add 10g of diethylamine, control the temperature at 5°C, stir for 60min until it dissolves, cool down to -40°C, add 13.2g of pivaloyl chloride, N - 0.83 g of methylmorpholine, reacted at -10°C for 60 min at a controlled temperature.
[0048] Add the solution to the mixed anhydride solution, add 3g of anhydrous sodium sulfate, control the temperature at -10°C, add diethylamine dropwise during the process to control the pH=8.5 and react for 2.5 hours to obtain a light yellow suspension, filter with suction, and the filtrate is at 40 ℃, distilled under -0.09Mpa vacuum condition until no distillate flowed out, then 240ml of water was added dropwise in 2 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com