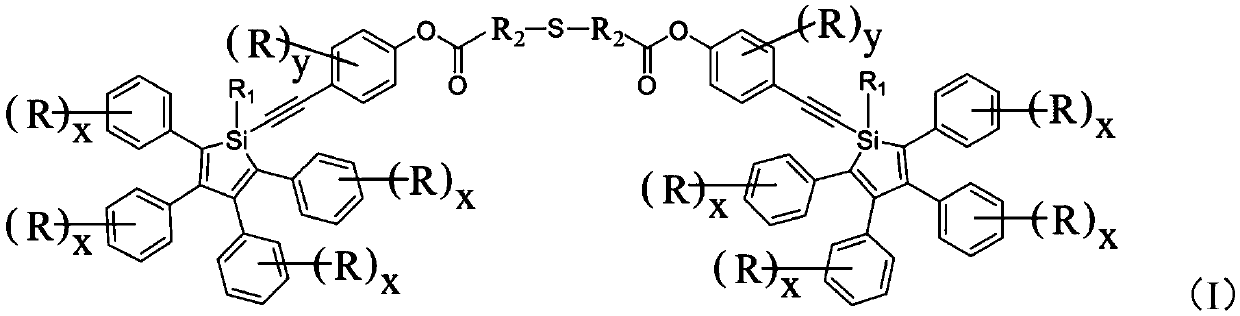
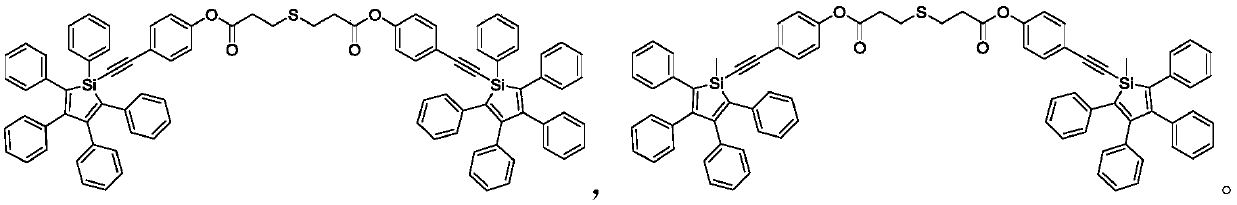
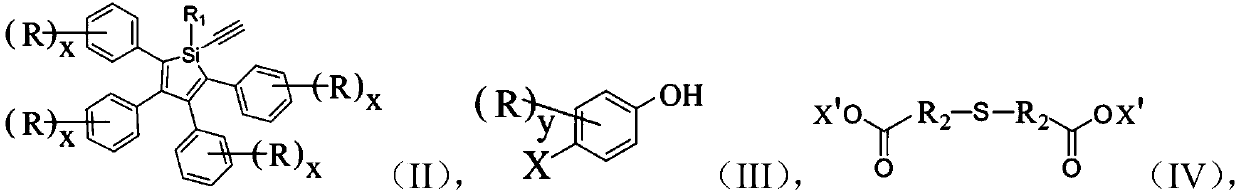
Silole derivative, preparation method and applications thereof, and photoluminescence lubricating grease
A technology of derivatives and lubricating grease, applied in the field of silole derivatives with luminescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Add 487mg (1mmol) 1-alkynyl-1,2,3,4,5-pentaphenylsilole, 264mg (1.2mmol) p-iodophenol, 19mg (0.1mmol) cuprous iodide in 100mL Schlenk reaction bottle, 26mg (0.1mmol) triphenylphosphine, 23mg (0.02mmol) tetrakistriphenylphosphine palladium was added under nitrogen protection, 30mL tetrahydrofuran / triethylamine (2 / 1, v / v), reacted at room temperature for 48 hours. After the reaction, filter and spin the filtrate to dryness, use dichloromethane / petroleum ether (1 / 1, v / v) mixed solvent as eluent to separate and purify the product by column chromatography to obtain 430 mg of yellow solid product, The yield was 74%. The NMR results of the product are: 1 HNMR (400MHz, CDCl 3 ), δ(TMS,ppm):7.74(m,2H),7.36(m,3H),7.15–6.85(m,24H); MS(MALDI-TOF):m / zcalcd:578.2[M] + , found: 578.2.
[0054] The reaction formula of embodiment 1 is as follows:
[0055]
Embodiment 2
[0057] Add 1158mg (2mmol) 1-(4-hydroxyphenynyl)-1,2,3,4,5-pentaphenylsilole, 178mg (1mmol) thiodipropionic acid, 630mg (2.4 mmol) triphenylphosphine, 30 mL tetrahydrofuran, 418 mg (2.4 mmol) diethyl azodicarboxylate was slowly added dropwise at 0° C., and then reacted at room temperature for 18 hours. After the reaction, filter and spin the filtrate to dryness, use dichloromethane / petroleum ether (1 / 2, v / v) mixed solvent as eluent to separate and purify the product by column chromatography to obtain 960 mg of yellow solid product, The yield was 78%. The NMR results of the product are: 1 H NMR (400MHz, CDCl 3 ), δ(TMS,ppm):7.72(m,4H),7.35(m,6H),7.13–6.85(m,48H),2.92(m,4H),2.78(m,4H); MS(MALDI- TOF):m / zcalcd:1298.4[M] + , found: 1298.4.
[0058] The reaction formula of embodiment 2 is as follows:
[0059]
Embodiment 3
[0061]In a 100mL Schlenk reaction flask, add 425mg (1mmol) 1-methyl-1-ynyl-2,3,4,5-tetraphenylsilole, 264mg (1.2mmol) p-iodophenol, 19mg (0.1mmol) iodide Cuprous, 26mg (0.1mmol) triphenylphosphine, under nitrogen protection, add 23mg (0.02mmol) tetrakistriphenylphosphine palladium, 30mL tetrahydrofuran / triethylamine (2 / 1, v / v), react at room temperature for 48 Hour. After the reaction, filter and spin the filtrate to dryness, use dichloromethane / petroleum ether (1 / 1, v / v) mixed solvent as eluent to separate and purify the product by column chromatography to obtain 400 mg of yellow solid product, The yield was 78%. The NMR results of the product are: 1 H NMR (400MHz, CDCl 3 ), δ(TMS,ppm):7.15–6.85(m,24H),0.22(s,3H); MS(MALDI-TOF):m / z calcd:516.2[M] + , found: 516.2.
[0062] The reaction formula of embodiment 3 is as follows:
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com