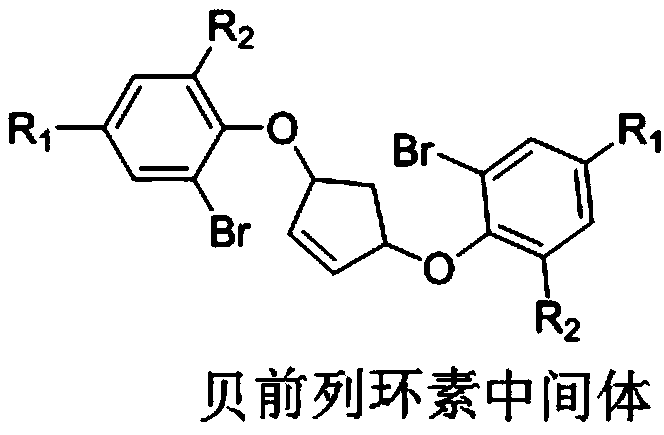
Preparation method for beraprost intermediate
A technology of beraprost and prostacyclin, which is applied in the field of preparation of beraprost intermediates, can solve the problems of high requirements for synthetic equipment and large pollution, and achieve reduced environmental protection treatment costs, reduced production costs, and fewer by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 prepares cyclopentadiene:
[0039] Depolymerization of cyclopentadiene:
[0040] Because of its conjugated diene and a methylene ring structure, cyclopentadiene is very active and prone to reactions such as polymerization, addition, and oxidation. It can be polymerized into dicyclopentadiene at room temperature, and it needs to be stored at -20°C, which limits its application. Usually, high-temperature depolymerization of dicyclopentadiene is used to produce cyclopentadiene.
[0041] Take 500g of dicyclopentadiene and put it into 200mg of 2,6-di-tert-butyl-4-methylphenol (BHT), heat it to 180-200°C for distillation with a thorn-type distillation head, and collect the fraction at 40-50°C. The second distillation was carried out, and BHT (5 mg) was added to the collected fractions after the distillation was completed, and 300 g of cyclopentadiene was collected, with a yield of 60%.
Embodiment 2
[0042] Example 2 Preparation of 3,5-dibromocyclopentene
[0043] The synthesis method of 3,5-dibromocyclopentene is as follows:
[0044] .
[0045] The preparation steps of 3,5-dibromocyclopentene are as follows:
[0046] Add 300g of cyclopentadiene to 120mL of chloroform in a cold well and cool down to -72°C. After the temperature drops to -72°C, dilute 726g of Br2 with 300mL of chloroform, and use a constant pressure dropping funnel to dilute the Br 2 The liquid is slowly added dropwise into the reaction system, and the temperature is controlled at -70°C during the dropwise addition, and the dropwise addition is completed;
[0047] The reaction system was naturally heated to 0°C, and saturated aqueous sodium bisulfite solution was added until the color of the solution faded, separated, the aqueous phase was extracted once with chloroform, the organic phase was combined, and the organic phase was washed once with saturated sodium bisulfite, and then washed with Wash once...
Embodiment 3
[0050] The difference between this example and Example 1 is that NBS (1620g) was diluted with 300mL chloroform and added dropwise to the reaction system as a bromine reagent, and finally 670g fraction was collected (the fraction was 3,5-dibromocyclopentene), producing rate of 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com