Adeno-associated virus vector carrying C3 gene expression cassette and application thereof
A gene expression and virus technology, applied in the field of recombinant adeno-associated virus vectors, can solve the problems of CAM promoters not having cell expression specificity, side effects of corneal edema, etc., and achieve the effect of increasing permeability, less damage, and improving discharge efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1 plasmid vector construction
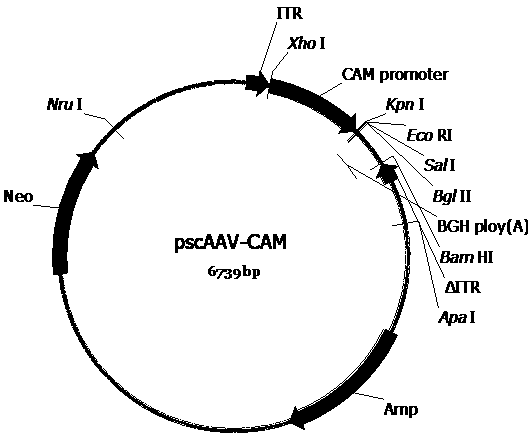
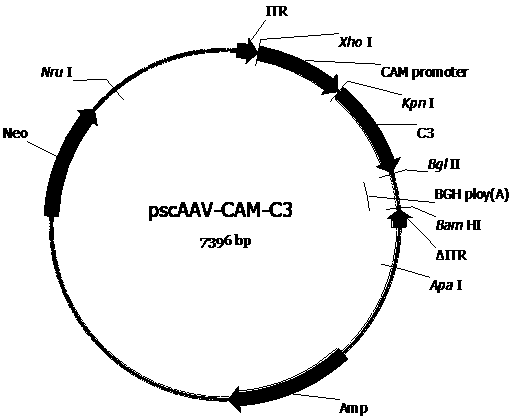
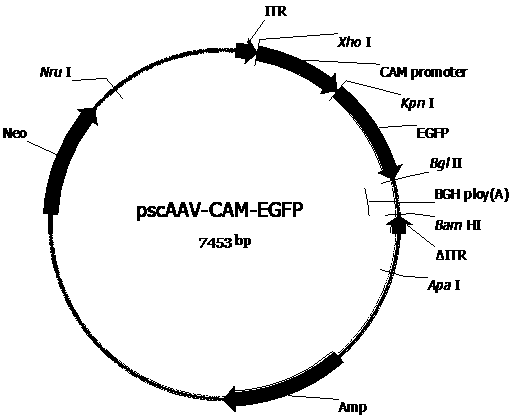
[0076] Construct the AAV vector plasmid (pscAAV-CAM-C3 (pscAAV-CAM-C3 ( image 3 ), pscAAV-CAM-EGFP ( Figure 4 ), pscAAV-CAM-Gluc ( Figure 5 ), pscAAV-CH3L1-Gluc ( Figure 6 ), pscAAV-CH3L1-C3 ( Figure 7 ), pscAAV-MGP-Gluc ( Figure 8 ), pscAAV-MGP-C3 ( Figure 9 )) for packaging preparation to obtain the required recombinant AAV virus. These plasmid vectors are in the pscAAV-CAM vector ( figure 2 ), the precursor plasmid of the pscAAV-CAM vector is pAAV2neo ( figure 1 ).
[0077] (1) Construction of pscAAV-CAM vector
[0078]To construct a double-stranded AAV vector, we first constructed a general-purpose pscAAV-CAM plasmid based on pAAV2neo. The construction process is as follows. Based on the 3' ITR sequence in the AAV2 genome (GenBank No.AF043303), the trs sequence and the D sequence in the ITR sequence were deleted according to literature reports (Wang Z, et al. Gene Ther. 2003; 10: 2105-2111.), to obtain t...
Embodiment 2
[0091] Embodiment 2 recombinant AAV virus preparation and assay
[0092] References (XiaoX, etal .JVirol.1998;72(3):2224-2232.), using the three-plasmid packaging system to package the recombinant AAV virus, and using cesium chloride density gradient centrifugation to separate, purify and package the AAV virus. Briefly, AAV vector plasmids (pscAAV-CAM-C3, pscAAV-CAM-EGFP, pscAAV-CAM-Gluc, pscAAV-CH3L1-C3, pscAAV-CH3L1-Gluc, pscAAV-MGP-C3, or pscAAV-MGP-Gluc), The helper plasmid (pHelper) and the AAV Rep and Cap protein expression plasmid (pAAV-R2C2) were mixed according to the molar ratio of 1:1:1, and the HEK293 cells were transfected by the calcium phosphate method. After 48 hours of transfection, the cells were harvested and cultured. The supernatant was separated and purified by cesium chloride density gradient centrifugation. Seven recombinant viruses including scAAV2-C3, scAAV2-EGFP, scAAV2-CAM-Gluc, scAAV2-CH3L1-C3, scAAV2-CH3L1-Gluc, scAAV2-MGP-C3, scAAV2-MGP-Gluc w...
Embodiment 3
[0110] Example 3 scAAV2-C3 virus evaluation experiment in vivo and in vitro
[0111] In order to verify whether the scAAV2-C3 virus can effectively transduce trabecular meshwork cells, express and produce C3 protein, reduce the stability of actin in cells, change the morphological structure of trabecular meshwork cells, and increase the permeability of trabecular meshwork, It is beneficial to the discharge of aqueous humor and lower intraocular pressure, so as to achieve the purpose of treating glaucoma. We designed a series of experiments. First, using scAAV2-EGFP as a control, we transduced primary cultured human trabecular meshwork cells with scAAV2-C3 virus, and observed changes in cell morphology and actin to verify the ability of scAAV2-C3 virus to transduce trabecular meshwork cells. Does it work. Next, we injected scAAV2-C3 virus into the eyes of C57BL / 6J mice through the anterior chamber, and observed the reduction of intraocular pressure in mice. Then, using scAAV-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com