Coated nickel-based catalyst and preparation method thereof
A nickel-based catalyst and encapsulated technology are applied in the field of encapsulated nickel-based catalysts and their preparation, which can solve the problems of insufficient hydrothermal stability of the Ni-based catalyst, and achieve the effects of controllable synthesis process and small grain size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
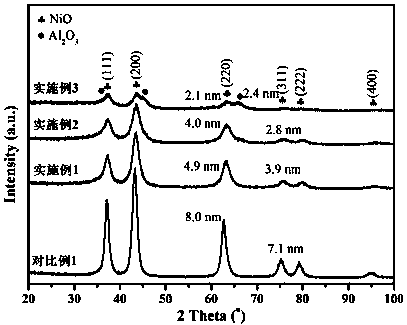
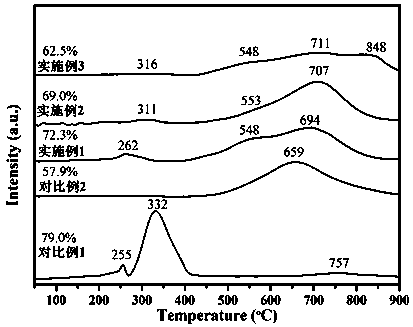
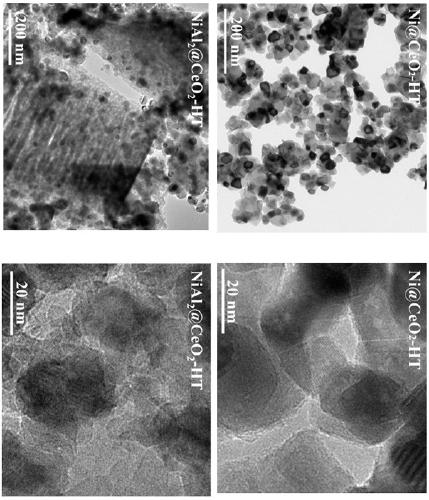
Examples
Embodiment 1
[0024] Preparation of oxidized nickel core NiAl 0.5 O: Weigh 1.32 g PEG (M W =20,000), 7.76 g Ni(NO 3 ) 2 ·6H 2 O and 5.00 g Al(NO 3 ) 3 9H 2 O was dissolved in 160 mL deionized water. Weigh 3.74 g of NaOH and dissolve in 400 mL of deionized water. The NaOH aqueous solution was added dropwise into the mixed nitrate aqueous solution, and after the dropwise addition was completed, stirring was continued for 1 h, and then placed in a 60 °C oven for hydrothermal reaction for 24 h. The obtained precipitate was centrifuged, washed with deionized water and ethanol, dried under vacuum at 50 °C for 24 h, and calcined at 450 °C for 4 h at a heating rate of 1 °C / min.
[0025] Preparation of NiAl 0.5 @CeO 2 -HT catalyst: Weigh 0.51 g oxidized nickel nucleus NiAl 0.5 O and 1.44 g Ce(NO 3 ) 3 ·6H 2 O was dispersed in 320 mL of 50% ethanol aqueous solution, ultrasonically dispersed for 1 h, and stirred overnight. Weigh 1.08 g of L-arginine and dissolve in 30 mL of deionized wa...
Embodiment 2
[0026] Example 2 Preparation of oxidized nickel core NiAlO: Weigh 1.32 g PEG (M W =20,000), 5.82 g Ni(NO 3 ) 2 ·6H 2 O and 7.50 g Al(NO 3 ) 3 9H 2 O was dissolved in 160 mL deionized water. Weigh 4 g of NaOH and dissolve in 400 mL of deionized water. The NaOH aqueous solution was added dropwise into the mixed nitrate aqueous solution, and after the dropwise addition was completed, stirring was continued for 1 h, and then placed in a 60 °C oven for hydrothermal reaction for 24 h. The obtained precipitate was centrifuged, washed with deionized water and ethanol, dried in vacuum at 50 °C for 24 h, and calcined at 450 °C for 4 h at a heating rate of 1 °C / min.
[0027] Preparation of NiAl@CeO 2 -HT catalyst: weigh 0.64 g NiAlO and 1.11 g Ce(NO 3 ) 3 ·6H 2 O was dispersed in 320 mL of 50% ethanol aqueous solution, ultrasonically dispersed for 1 h, and stirred overnight. Weigh 0.83 g of L-arginine and dissolve in 30 mL of deionized water. At room temperature, the L-argin...
Embodiment 3
[0029] Preparation of oxidized nickel core NiAl 2 O: Weigh 1.32 g PEG (M W =20,000), 3.88 g Ni(NO 3 ) 2 ·6H 2 O and 10.00 g Al(NO 3 ) 3 9H 2 O was dissolved in 160 mL deionized water. Weigh 4.27 g of NaOH and dissolve in 400 mL of deionized water. The NaOH aqueous solution was added dropwise into the mixed nitrate aqueous solution, and after the dropwise addition was completed, stirring was continued for 1 h, and then placed in a 60 °C oven for hydrothermal reaction for 24 h. The obtained precipitate was centrifuged, washed with deionized water and ethanol, dried under vacuum at 50 °C for 24 h, and calcined at 450 °C for 4 h at a heating rate of 1 °C / min.
[0030] Preparation of NiAl 2 @CeO 2 -HT catalyst: Weigh 0.90 g oxidized nickel core NiAl 2 O and 0.45 g Ce(NO 3 ) 3 ·6H 2 O was dispersed in 320 mL of 50% ethanol aqueous solution, ultrasonically dispersed for 1 h, and stirred overnight. Weigh 0.34 g of L-arginine and dissolve in 30 mL of deionized water. At...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com