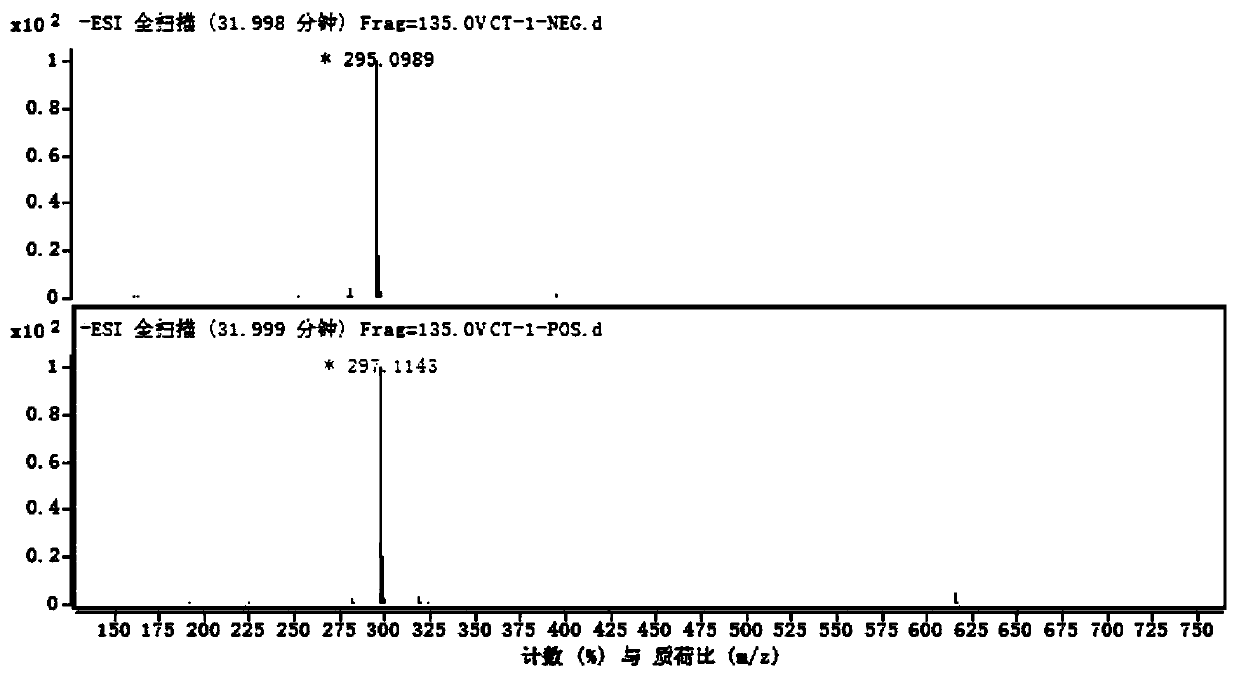
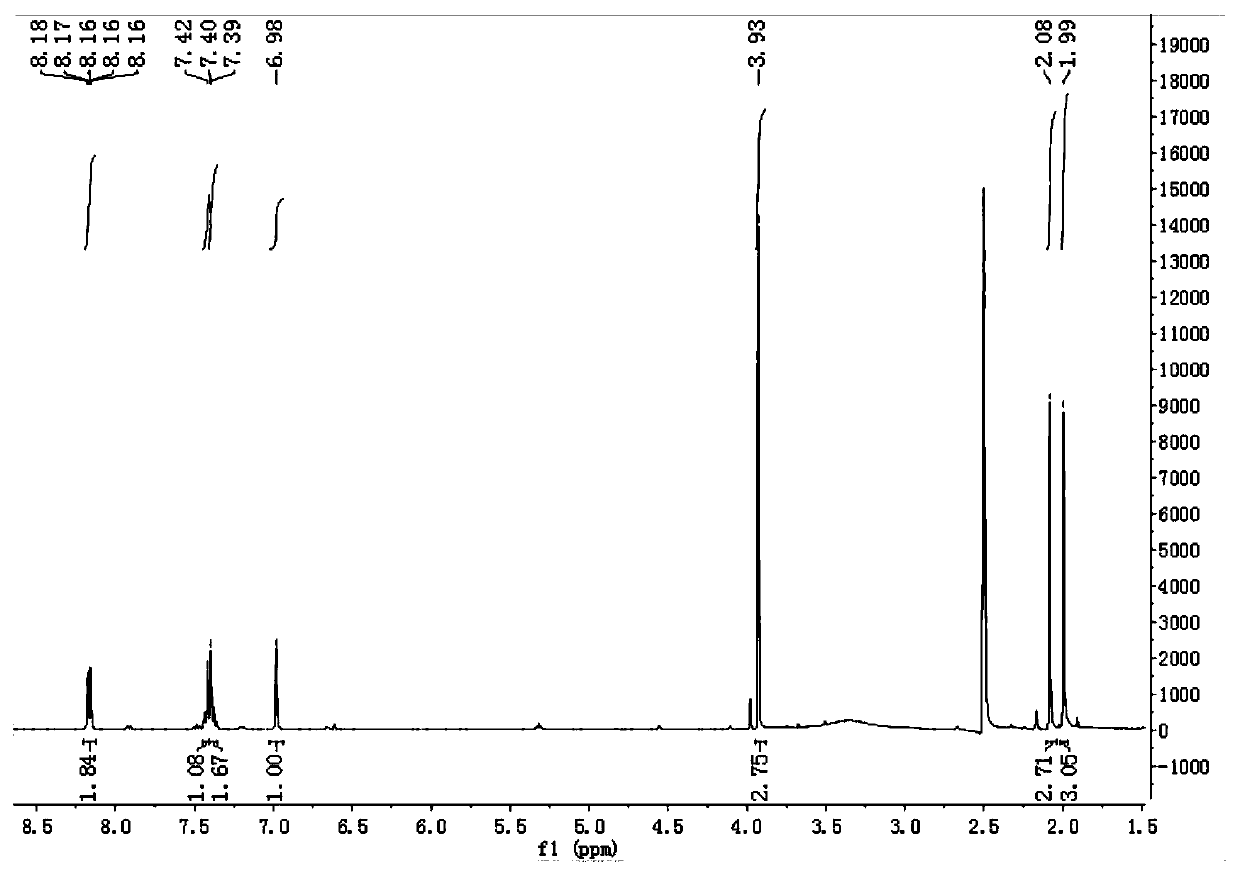
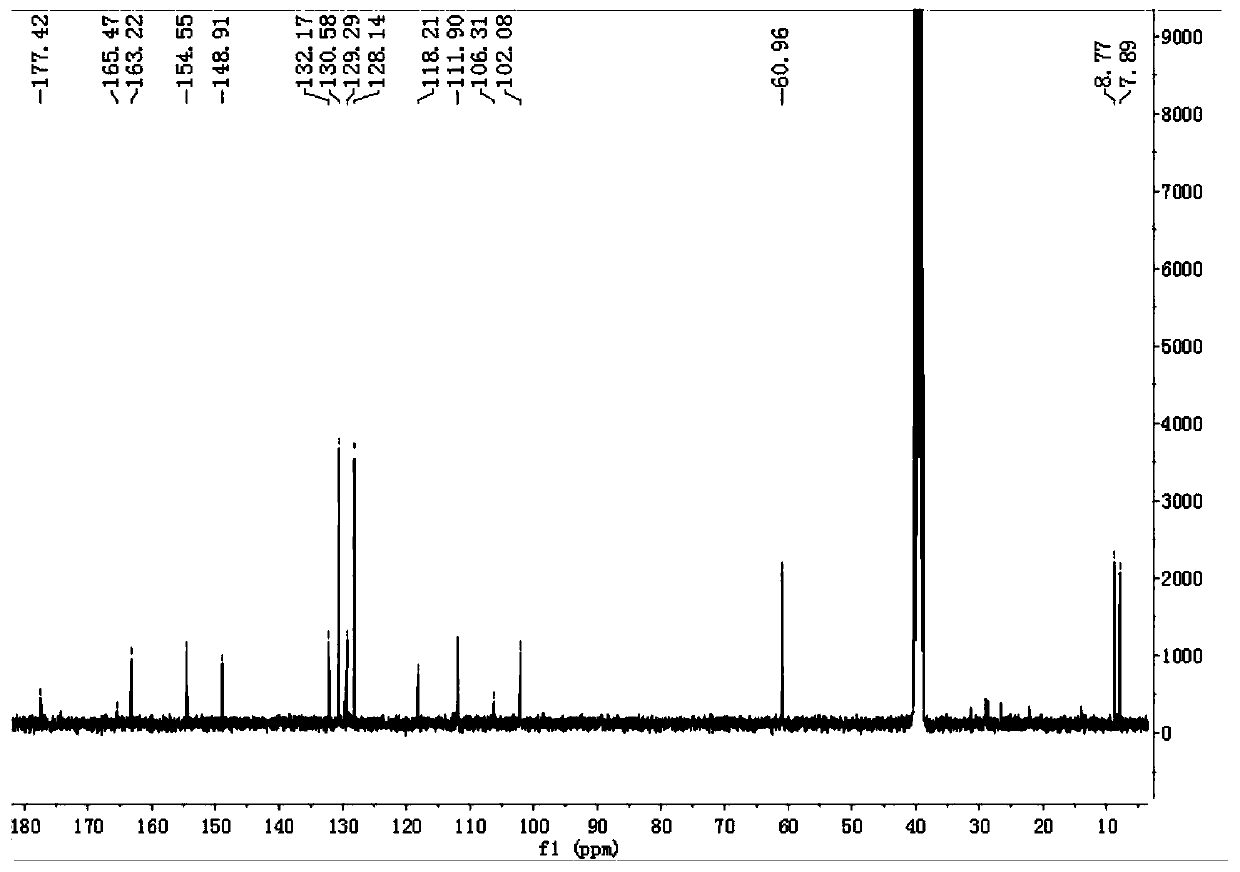
Compound with anti-inflammatory effect and preparation method and application thereof
An anti-inflammatory effect and compound technology, applied in the field of medicine, can solve problems such as complex components, difficult to clarify the material basis and mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 compound of the present invention
[0041] 1) 5Kg of dried water wenghua, add 10 times the volume concentration of 70% ethanol aqueous solution, extract twice, each time for 2h, to obtain the extract;
[0042] 2) Add an appropriate amount of purified water to the extract of step 1) to adjust the volume concentration of ethanol to 60%, let it stand overnight, take the supernatant and absorb it with D101 macroporous resin, and then elute with 60% ethanol for 4 column volumes to remove impurities , and then 4 column volumes were eluted with 90% ethanol, and the 90% ethanol eluate was collected and concentrated under reduced pressure to recover the solvent to obtain an extract;
[0043] 3) Dissolve the extract in step 2) with ethyl acetate, add petroleum ether and stir, wherein the volume ratio of ethyl acetate to petroleum ether is 1:10; take the supernatant to recover the solvent to obtain the extract;
[0044] 4) The medicinal extract in st...
Embodiment 2
[0057] The preparation of embodiment 2 compounds of the present invention
[0058] 1) 5Kg of dried water wenghua, add 7 times the volume concentration of 80% ethanol aqueous solution, extract 3 times, each time for 1.5h, to obtain the extract;
[0059] 2) Add appropriate amount of purified water to the extract of step 1) to adjust the volume concentration of ethanol to 50%, let it stand overnight, take the supernatant and absorb it through HP-20 macroporous resin, and then elute with 70% ethanol for 4 column volumes Remove impurities, then elute 4 column volumes with 95% ethanol, collect the 95% ethanol eluate and concentrate under reduced pressure to recover the solvent to obtain an extract;
[0060] 3) Dissolve the extract in step 2) with ethyl acetate, add petroleum ether and stir, wherein the volume ratio of ethyl acetate to petroleum ether is 1:15; take the supernatant to recover the solvent to obtain the extract;
[0061] 4) The medicinal extract in step 3) is separated...
Embodiment 3
[0063] The preparation of embodiment 3 compounds of the present invention
[0064] 1) 5Kg of dried water wenghua, add 8 times the volume concentration of 90% ethanol aqueous solution, extract once, each time for 1h, to obtain the extract;
[0065] 2) Take the extract of step 1) and let it stand overnight, take the supernatant and absorb it through AB-8 macroporous resin, then use 50% ethanol to elute 4 column volumes to remove impurities, and then use 80% ethanol to elute 4 columns Volume, collect 80% ethanol eluate and concentrate under reduced pressure to recover the solvent to obtain extract;
[0066] 3) Dissolve the extract in step 2) with ethyl acetate, add petroleum ether and stir, wherein the volume ratio of ethyl acetate to petroleum ether is 1:5; take the supernatant to recover the solvent to obtain the extract;
[0067] 4) The medicinal extract in step 3) is separated by preparative HPLC, and the volume concentration is 60% acetonitrile-0.1% formic acid aqueous solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com