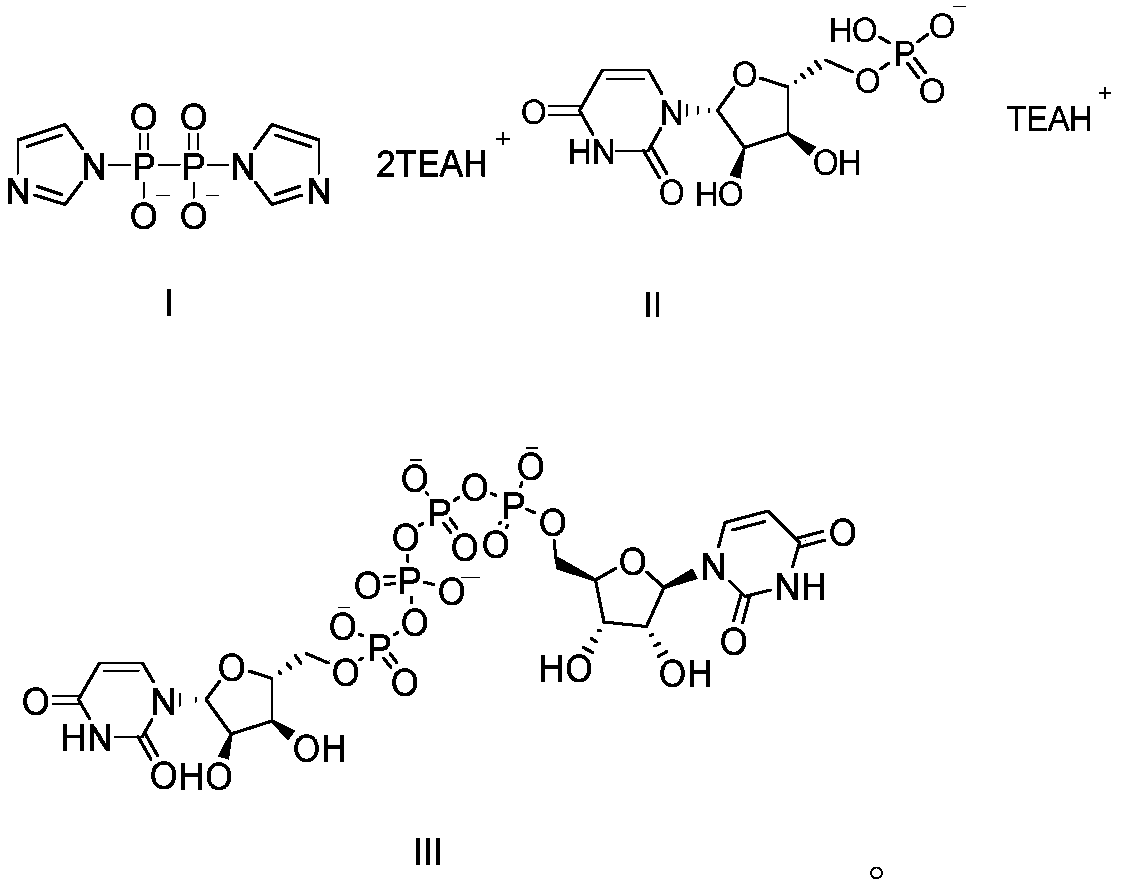
Preparation method of P<1>,P<4>-di(uridine 5'-)tetraphosphate
A technology of uridine monophosphate triethylamine salt and tetraphosphate, which is applied in the field of P1, can solve the problems of non-residual metal ion research and difficulty in reaching pharmaceutical standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] a) Preparation of bistriethylamine pyrophosphate
[0058] Put the pretreated hydrogen-type cation exchange resin PK216 (2.4L) into the chromatography column, add disodium pyrophosphate (75.2g, 282.81mmol) into 1.8L water and stir to dissolve, add the aqueous solution to the hydrogen-type cation-exchange resin In PK216, after soaking for 1 hour, elute with purified water to pH 5.5-6.5, combine the eluents, and titrate the content of pyrophosphoric acid (43.2g, 242.24mmol) and add triethylamine (50.7g, 513.26mmol), Stir for 30min, concentrate under reduced pressure at 45-50°C until white semi-solid, add appropriate amount of N,N'-dimethylformamide (500mL) to the residue for azeotropic dehydration, after concentration is complete, add N,N'- Dimethylformamide, stir evenly to obtain N,N'-dimethylformamide suspension of bistriethylamine pyrophosphate.
[0059] b) Preparation of uridine triethylamine salt
[0060] Put the pretreated hydrogen-form cation exchange resin PK216 ...
Embodiment 2
[0068] a), b), c) steps are the same as in Example 1.
[0069] d)P 1 , P 4 Preparation of Di(uridine 5'-)tetraphosphate
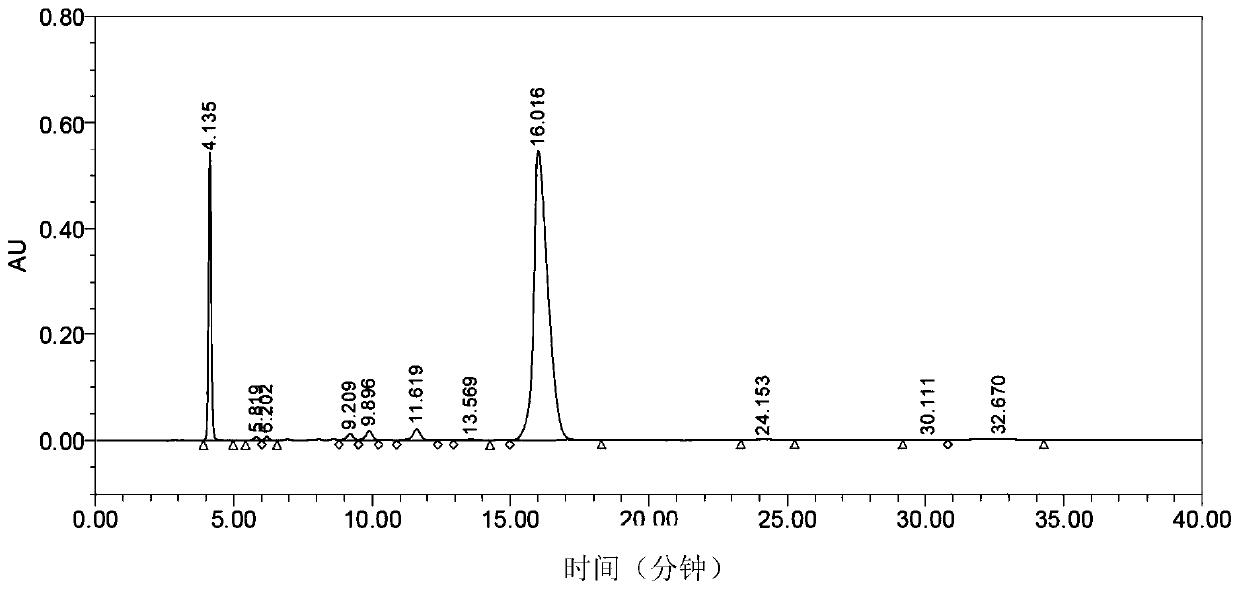
[0070] Mix the N,N'-dimethylformamide solution of uridine acid triethylamine salt obtained in step b) and the N,N'-dimethylformamide solution of imidazole pyrophosphate triethylamine salt obtained in step c) , stirred evenly, added anhydrous manganese chloride (306.2g, 2.43mol) in batches, and controlled the reaction temperature between 25-35°C. After the addition is complete, the mixture is kept under the condition of 25-35° C. and stirred for 2 hours. HPLC detection.
[0071] e) The steps are the same as in Example 1.
Embodiment 3
[0073] a), b), c) steps are the same as in Example 1.
[0074] d)P 1 , P 4 Preparation of Di(uridine 5'-)tetraphosphate
[0075] Mix the N,N'-dimethylformamide solution of uridine acid triethylamine salt obtained in step b) and the N,N'-dimethylformamide solution of imidazole pyrophosphate triethylamine salt obtained in step c) , stirred evenly, added anhydrous manganese chloride (153.5g, 1.22mol) in batches, and controlled the reaction temperature between 25-35°C. After the addition was complete, the mixture was incubated and stirred for 4 hours under the condition of 25-35°C. HPLC detection.
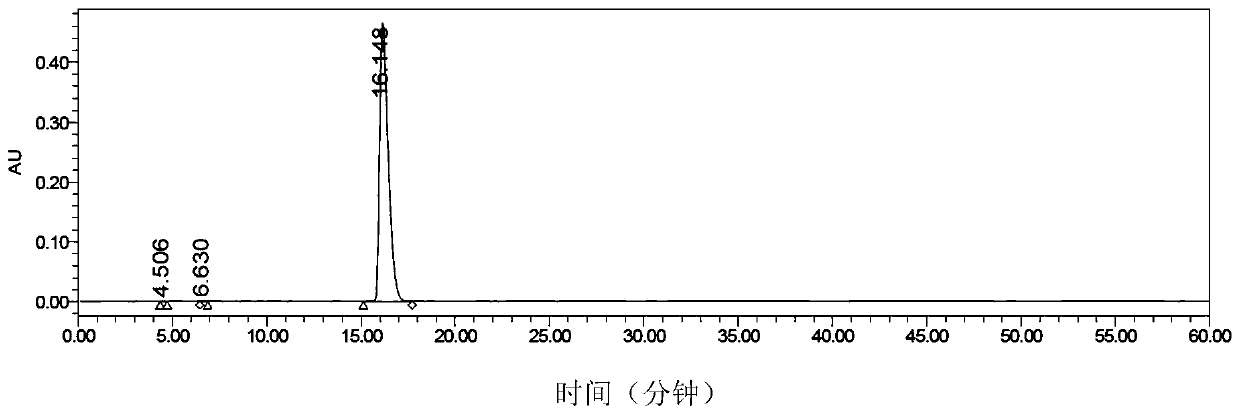
[0076] e)P 1 , P 4 Purification of Di(uridine 5'-)tetraphosphate
[0077] After the reaction was completed, the reaction solution was stirred with 1.8L ethyl acetate, filtered, the solid was dissolved in 1000mL, solid sodium bicarbonate (102.5g, 1.22mol) and sodium carbonate (64.65g, 610mmol) were added, stirred for 2 hours, filtered, the filtrate Add concentrated hydrochloric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com