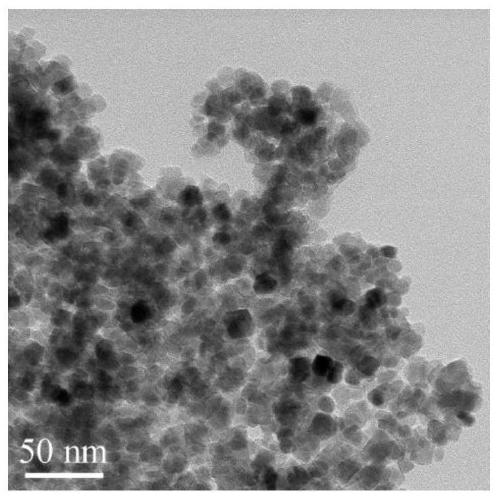
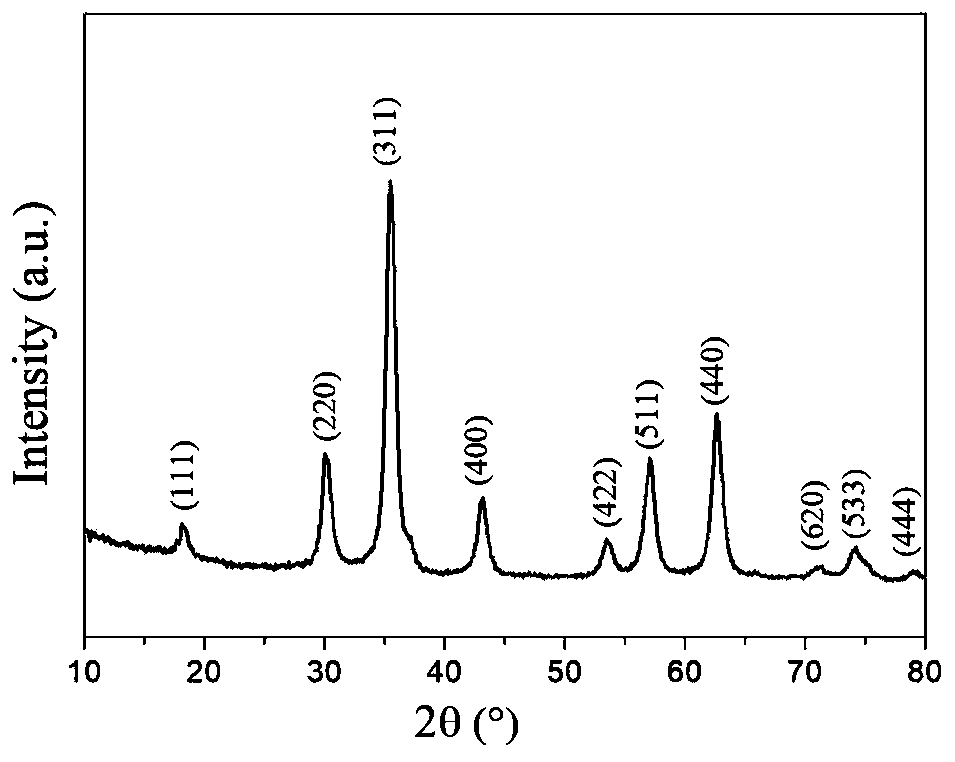
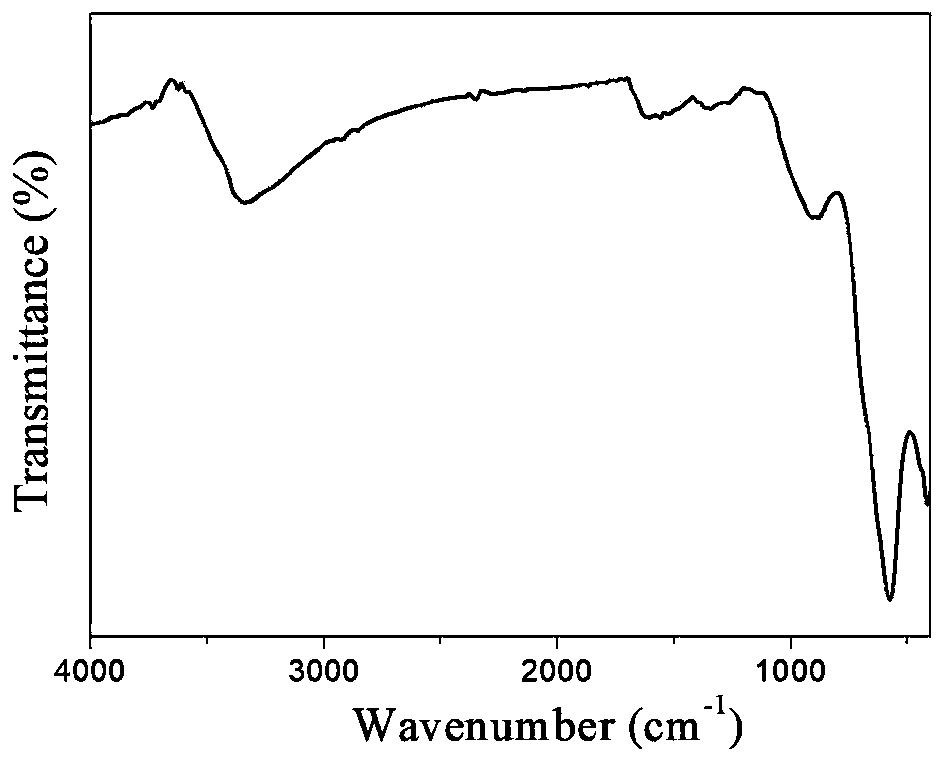
Magnetic copper (II) chelating ferroferric oxide @carbon nanoparticles and preparation method thereof, and method for immobilizing laccase
A technology of ferric tetroxide and carbon nanoparticles, which is applied in biochemical equipment and methods, oxidoreductase, immobilized on or in inorganic carriers, etc. problems such as low volume, to achieve the effect of high yield, easy recycling, and large quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A preparation method of magnetic copper (II) chelated ferric oxide@carbon nanoparticles, comprising the following steps:
[0059] 1) Preparation of hydroxyl / carboxyl functionalized magnetic Fe 3 o 4 The operation process of @C nanoparticles is as follows:
[0060] 1-1) In a three-necked flask, dissolve 0.4mmol of ferrous chloride and 0.8mmol of ferric chloride in 50mL of water, and add 4mL of concentrated ammonia (28% concentration by mass) under stirring in a nitrogen atmosphere. Add ammonia water 3 times; first add 2mL concentrated ammonia water, react at 60°C for 20min, then add 1mL concentrated ammonia water at 60°C, react at 60°C for 20min, then add 1mL concentrated ammonia water, ℃ for 20 minutes, and finally heated up to 90 ℃ for 1 hour to prepare Fe 3 o 4 Nanoparticle suspension, cooled to room temperature;
[0061] 1-2) To the above Fe 3 o 4 After adding 9mmol glucose and 180mmol sodium hydroxide to the nanoparticle suspension, transfer it to the autocla...
Embodiment 2
[0072] A preparation method of magnetic copper (II) chelated ferric oxide@carbon nanoparticles, comprising the following steps:
[0073] 1) Preparation of hydroxyl / carboxyl functionalized magnetic Fe 3 o 4 The operation process of @C nanoparticles is as follows:
[0074] 1-1) In a three-necked flask, dissolve 3mmol of ferrous chloride and 3mmol of ferric chloride in 100mL of water, and add 4mL of concentrated ammonia water (28% by mass concentration) under stirring in a nitrogen atmosphere, and 4mL of concentrated ammonia water Add 3 times; first add 2mL concentrated ammonia water, react at 70°C for 20min, then add 1mL concentrated ammonia water at 70°C, react at 70°C for 20min, then add 1mL concentrated ammonia water, The reaction was carried out at 100°C for 80 minutes, and the temperature was raised to 100°C for 2 hours to prepare Fe 3 o 4 Nanoparticle suspension, cooled to room temperature;
[0075] 1-2) To the above Fe 3 o 4 After adding 12mmol sucrose and 180mmol ...
Embodiment 3
[0083] A preparation method of magnetic copper (II) chelated ferric oxide@carbon nanoparticles, comprising the following steps:
[0084] 1) Preparation of hydroxyl / carboxyl functionalized magnetic Fe 3 o 4 The operation process of @C nanoparticles is as follows:
[0085] 1-1) In a three-necked flask, dissolve 0.4mmol of ferrous chloride and 0.8mmol of ferric chloride in 50mL of water, and add 4mL of concentrated ammonia (28% concentration by mass) under stirring in a nitrogen atmosphere. Add ammonia water 3 times; first add 2mL concentrated ammonia water, react at 60°C for 20min, then add 1mL concentrated ammonia water at 60°C, react at 60°C for 20min, then add 1mL concentrated ammonia water, ℃ for 20 minutes, and finally heated up to 90 ℃ for 1 hour to prepare Fe 3 o 4 Nanoparticle suspension, cooled to room temperature;
[0086] 1-2) To the above Fe 3 o 4 After adding 9mmol glucose and 180mmol sodium hydroxide to the nanoparticle suspension, transfer it to the autocla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Loading capacity | aaaaa | aaaaa |
| Loading capacity | aaaaa | aaaaa |
| Loading capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com