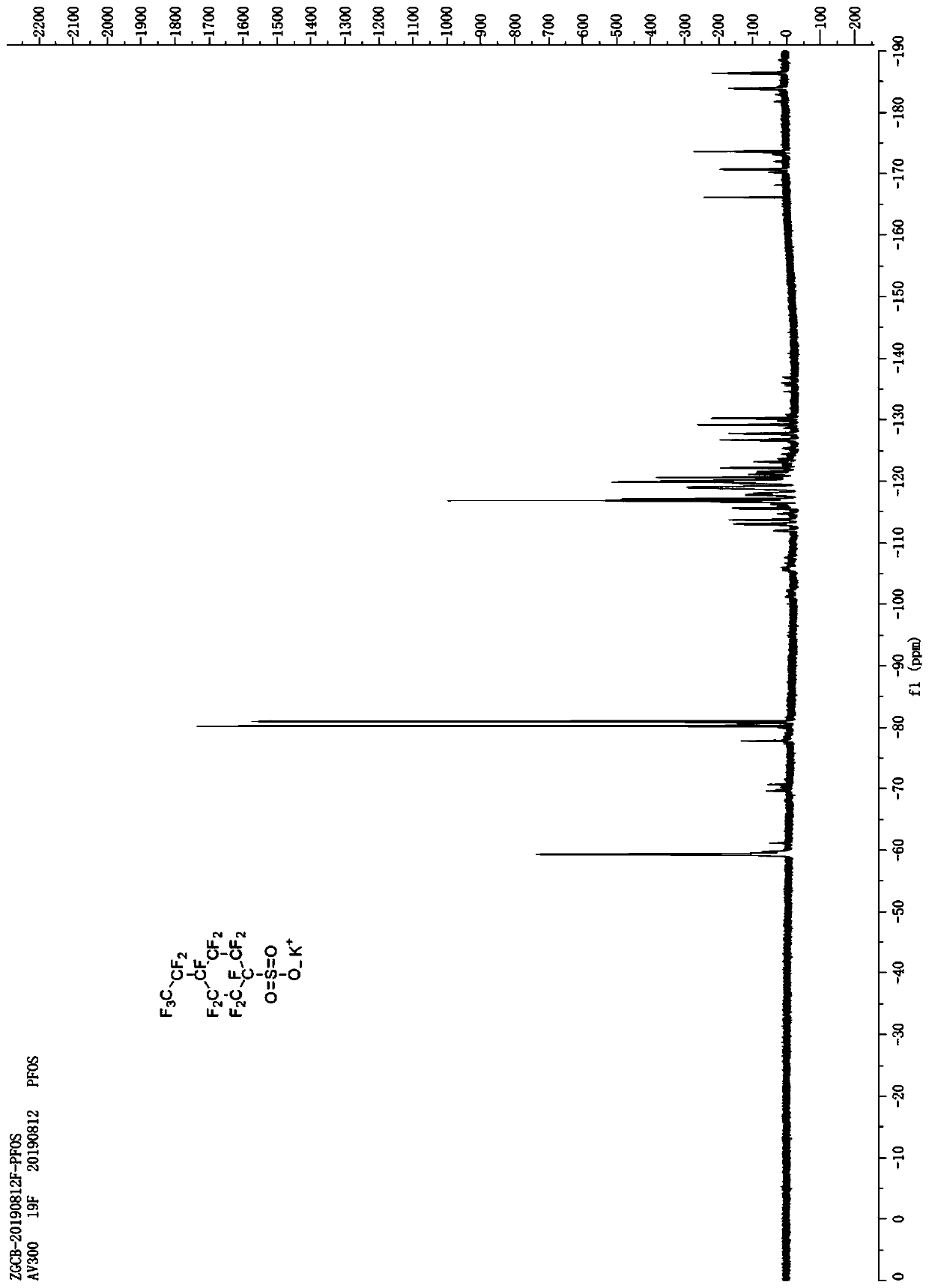
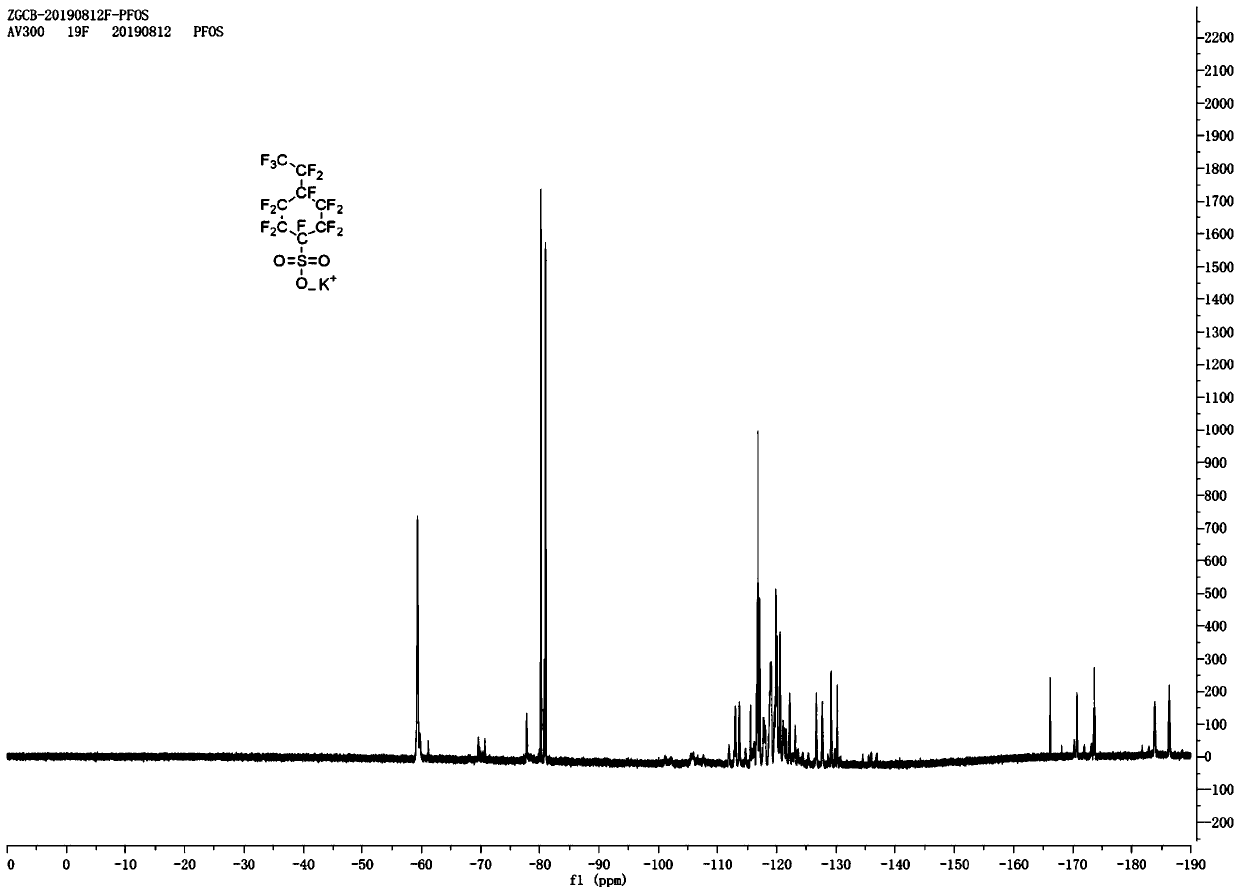
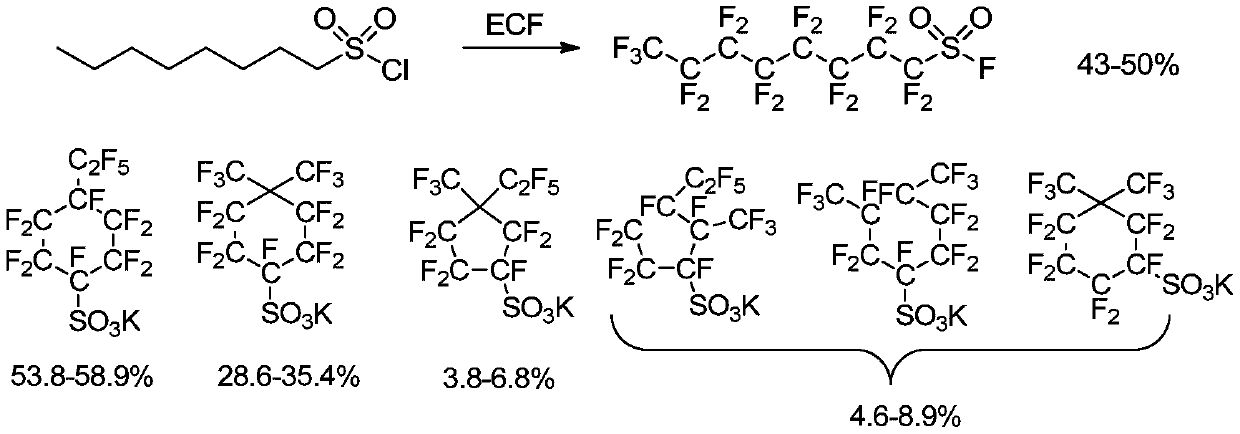
Preparation method and application of potassium decafluoro-4-(pentafluoroethyl)cyclohexyl-1-sulfonate
The technology of pentafluoroethyl and cyclohexyl is applied in the field of preparation and application of potassium decafluoro-4-cyclohexyl-1-sulfonate, and can solve the problem that the yield is only 25% and the cyclic perfluoroalkanesulfonic acid is only 25%. Problems such as loss of access to potassium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) React p-ethylbenzenesulfonyl chloride and potassium fluoride in acetonitrile. Control the reaction temperature at (80±5)°C. After 8 hours of reaction, the reaction system will naturally return to room temperature. First filter to remove solid impurities, and then carry out distillation collection p-ethylbenzenesulfonyl fluoride;
[0026] (2) The p-ethylbenzenesulfonyl fluoride collected in step (1) is added in the anhydrous hydrogen fluoride electrolyzer, and n-butanethiol is added in the electrolytic solution (hydrogen fluoride and potassium fluoride) and n-butanethiol is electrolyzed The mass fraction in the liquid is 0.5%, the temperature of the electrolyte is controlled at 0°C, a voltage of 5.5V is applied to the electrode for electrolysis, and the electrolysis is carried out for 8 hours. The electrolysis product is collected by liquid separation to obtain decafluoro-4-(perfluoroethyl) ring Crude hexyl-1-sulfonyl fluoride;
[0027] (3) The crude product of deca...
Embodiment 2
[0032] (1) React p-ethylbenzenesulfonyl chloride and cesium fluoride in dimethyl sulfoxide. Control the reaction temperature at (60±5)°C. After 8 hours of reaction, the reaction system will naturally return to room temperature, and first filter to remove solid impurities. Carry out distillation again and collect p-ethylbenzenesulfonyl fluoride;
[0033] (2) The p-ethylbenzenesulfonyl fluoride collected in step (1) is added in the anhydrous hydrogen fluoride electrolyzer, and n-butanethiol is added in the electrolytic solution (hydrogen fluoride and potassium fluoride) and n-butanethiol is electrolyzed The mass fraction in the solution is 0.6%, the temperature of the electrolyte is controlled at 5°C, and a voltage of 5V is applied to the electrode for electrolysis. After electrolysis for 12 hours, the electrolysis product is collected by liquid separation to obtain decafluoro-4-(perfluoroethyl)cyclohexyl - Crude 1-sulfonyl fluoride;
[0034] (3) The crude product of decafluoro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com