Preparation method of 2-methylquinoline
A technology of methylquinoline and nitrobenzene, which is applied in the field of preparation of 2-methylquinoline, can solve the problems of complex process, unfriendly environment, harsh reaction conditions, etc., and achieve less reaction equipment and simple and easy preparation method , the effect of less preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Accurately weigh 0.005mmol palladium acetate catalyst, add 10mL into the Young's reaction tube which has been placed in the magnetic stirrer, replace the oxygen in the Young's reaction tube three times, make the reaction proceed under the oxygen condition, and accurately add into the Young's reaction tube with a syringe 0.4mmol silver tetrafluoroborate, 0.1mmol pivalic acid, 0.2mmol nitrobenzene and 1ml ethanol, put the above-mentioned Young's reaction tube on a magnetic stirrer, and stir at 180°C for 24h; after the reaction, column the reaction solution The pure product of 2-methylquinoline was obtained by chromatography with a yield of 81%.
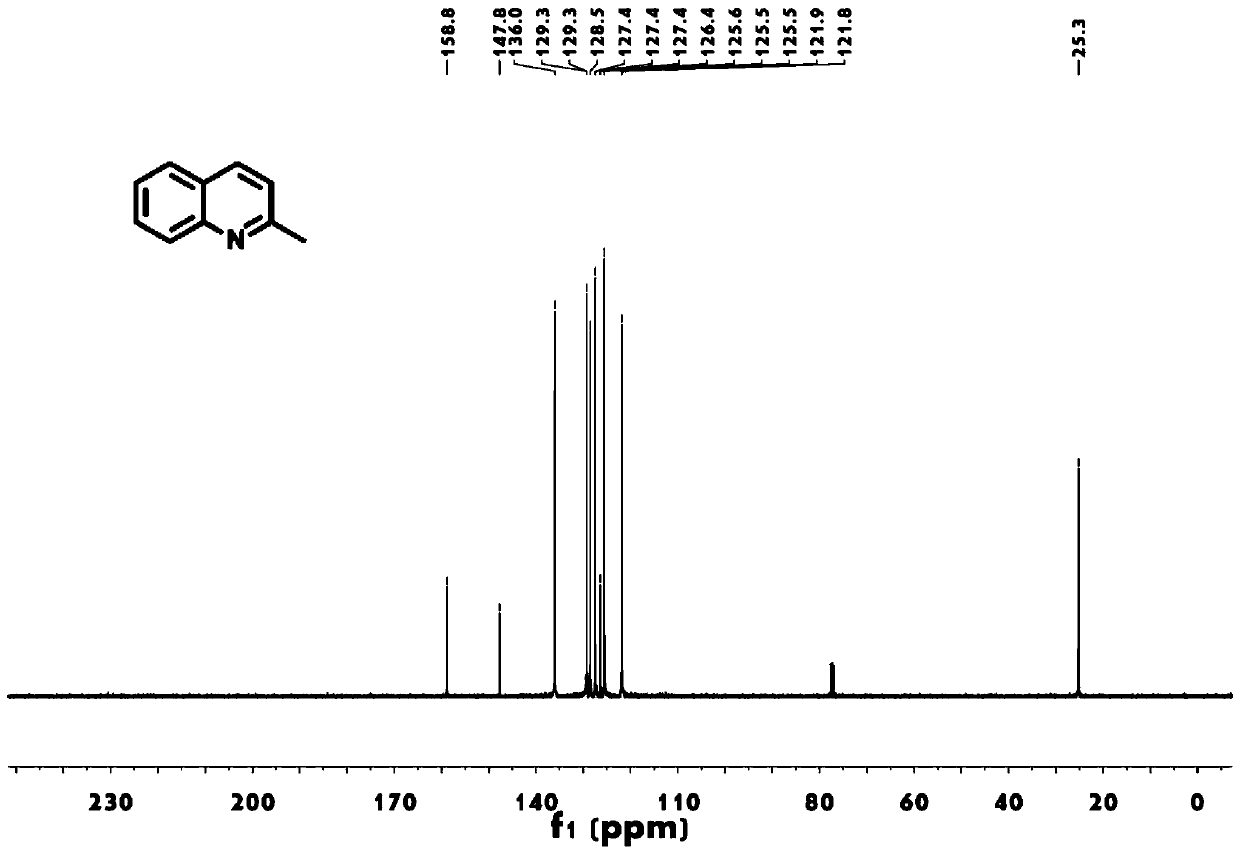
[0030] The above-mentioned obtained 2-methylquinoline has been characterized and tested:
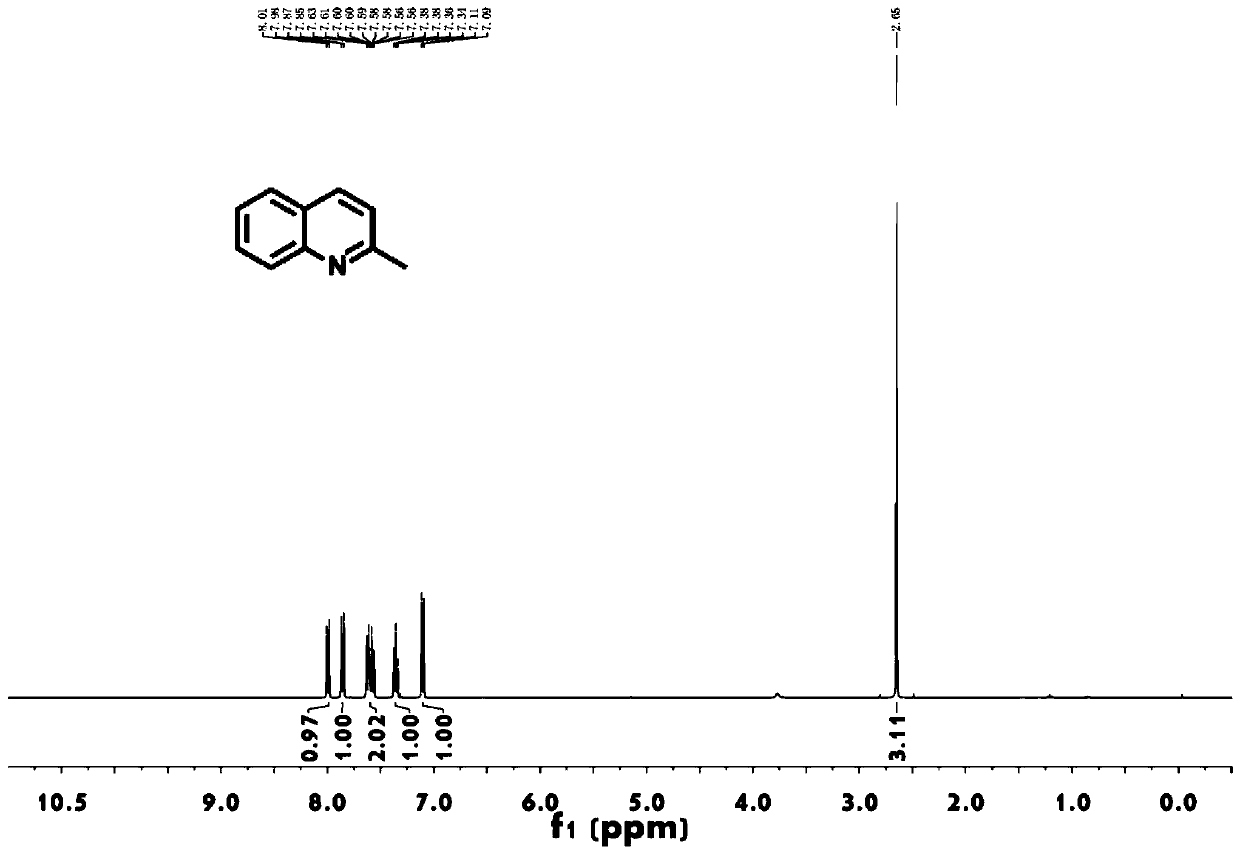
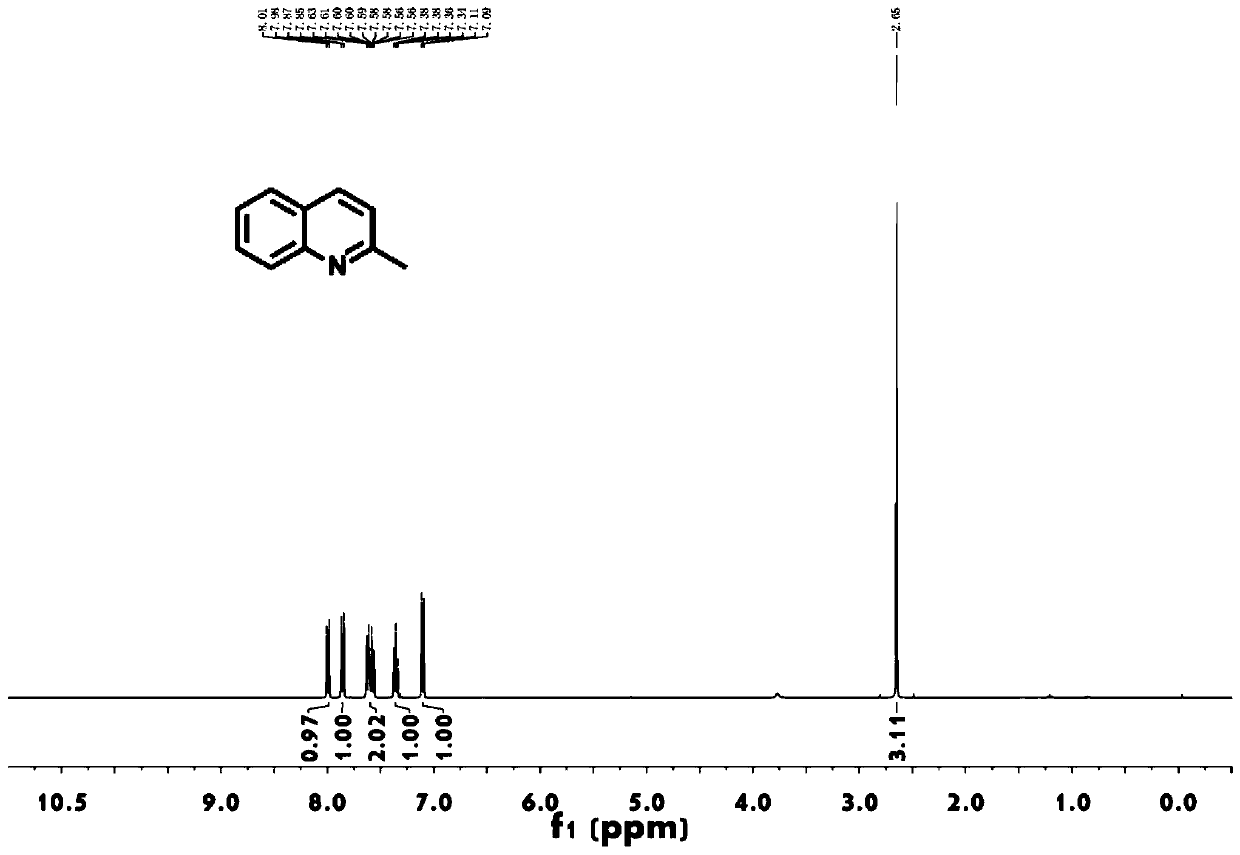
[0031] as attached figure 1 Shown, the 2-methylquinoline prepared by this method 1 H-NMR, its compound characterization data: 1 H-NMR (400MHz, CDCl 3 ): δ (ppm) = 2.65 (s, 3.0H), 7.10 (d, J = 8.0Hz, 1.0H), 7.38 (m, 1.0H), 7.58 (m, 1.0H)...
Embodiment 2
[0035] Accurately weigh 0.005mmol of palladium acetate catalyst, add 10mL into the Young's reaction tube which has been placed in a magnetic stirrer, and accurately add 0.4mmol of silver tetrafluoroborate, 0.1mmol of pivalic acid, and 0.2mmol of nitrobenzene into the Young's reaction tube with a syringe and 1ml of ethanol, the above-mentioned Young's reaction tube was placed on a magnetic stirrer, and stirred at 100°C for 24h; after the reaction, the reaction solution was subjected to column chromatography to obtain the pure product of 2-methylquinoline, with a yield of 32%. .
Embodiment 3
[0037] Accurately weigh 0.01mmol of palladium acetate catalyst, add 10mL into the Young's reaction tube which has been placed in the magnetic stirrer, replace the oxygen in the Young's reaction tube three times, make the reaction proceed under the oxygen condition, and accurately add into the Young's reaction tube with a syringe 0.4mmol silver acetate, 0.2mmol trifluoroacetic acid, 0.2mmol nitrobenzene and 1ml ethanol, put the above-mentioned Young's reaction tube on a magnetic stirrer, stir at 140°C for 24h; after the reaction, perform column chromatography on the reaction solution The pure product of 2-methylquinoline was obtained with a yield of 68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com