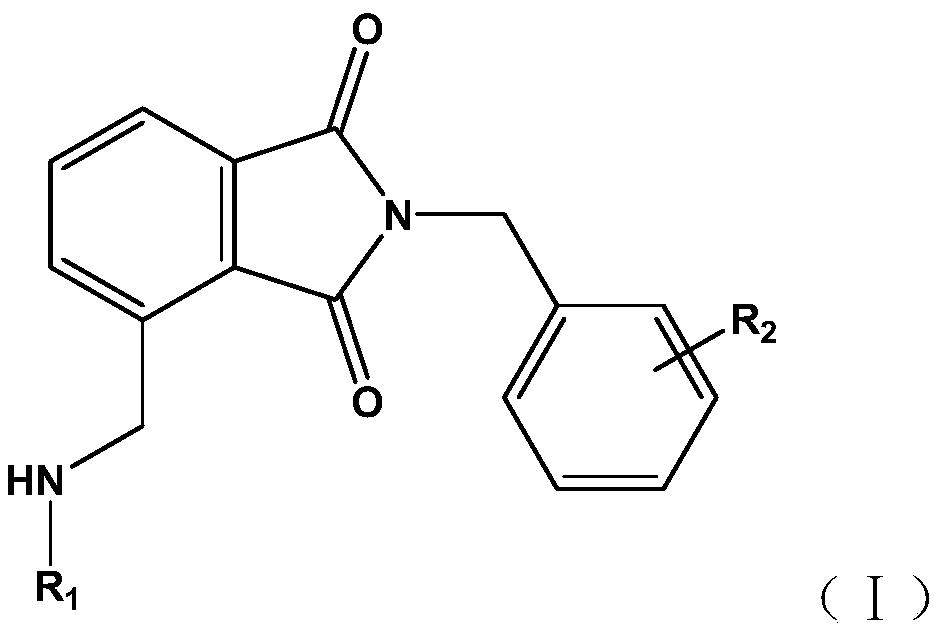
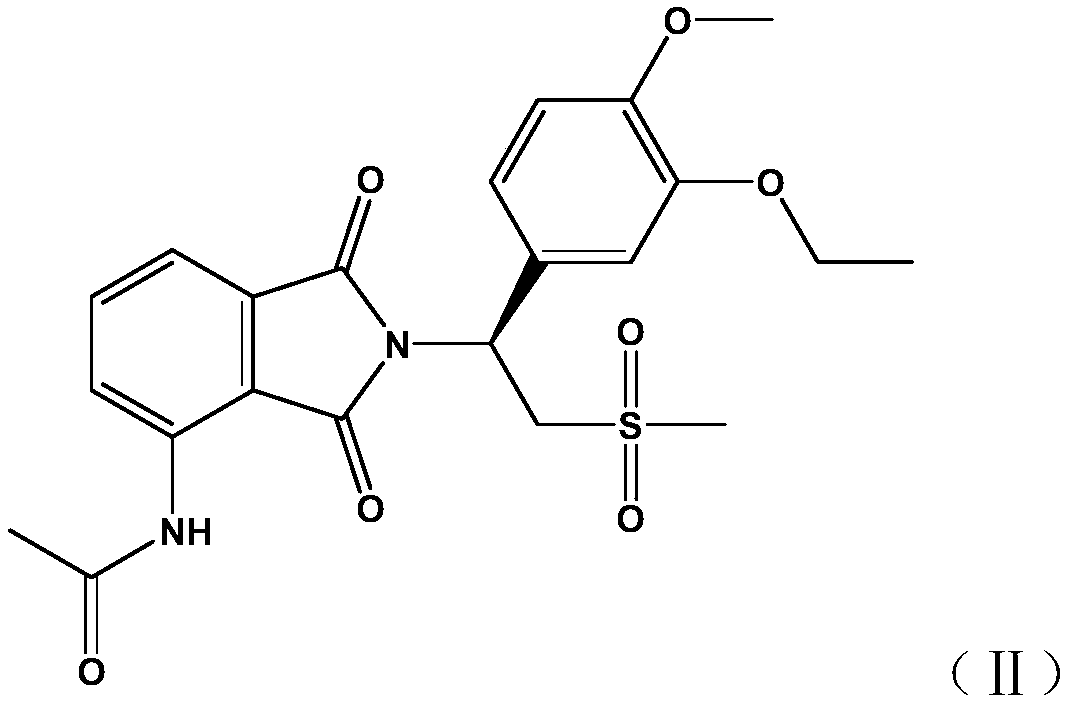
Phthalimide compound used as PDE2/4 dual inhibitor and preparation method thereof
A phthalimide and dual-inhibitor technology, applied in the field of phthalimide compounds and their preparation, can solve the problem of poor pharmacokinetic properties, weak and side effects of PDEs inhibitors Effectiveness and other issues, to achieve a good effect of dual inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
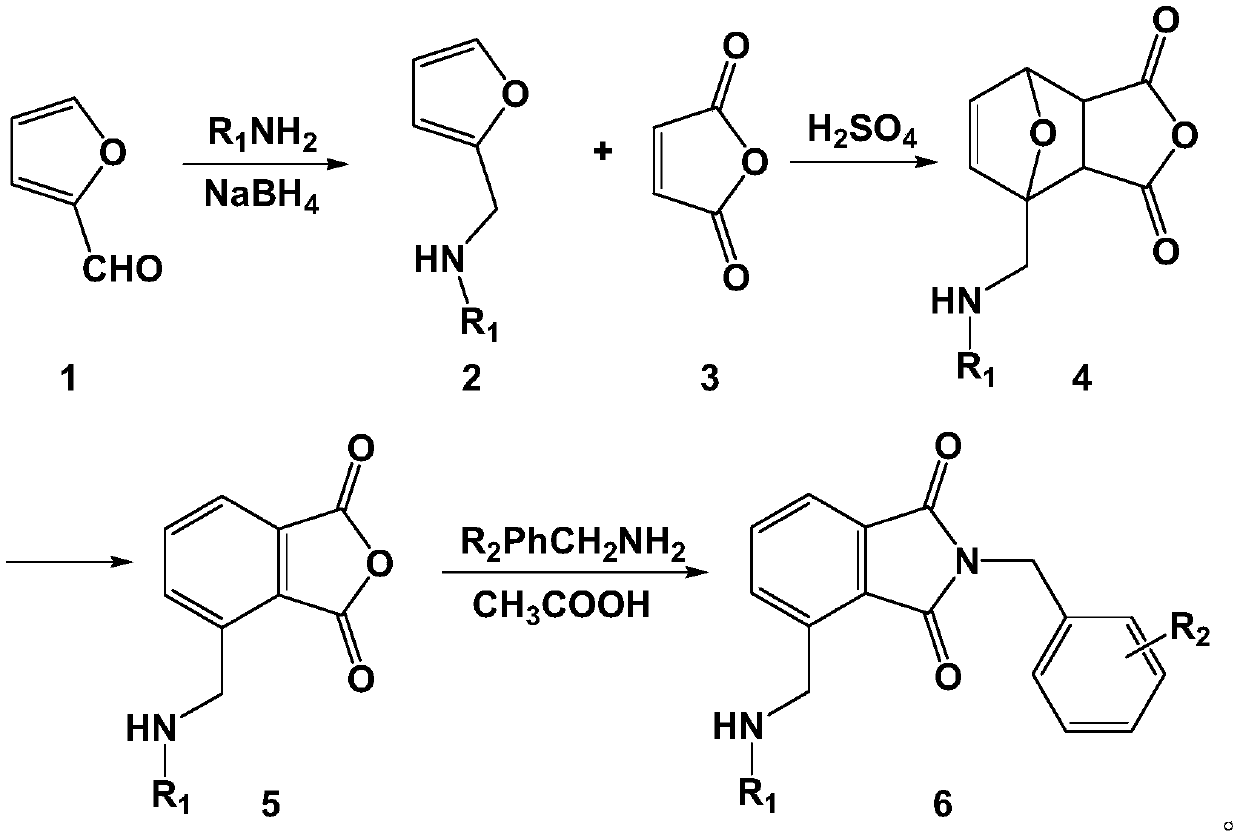
[0024] Embodiment 1: the preparation of compound a
[0025] 1. Preparation of Intermediate 2
[0026]
[0027] Add 20ml of a mixed solvent of dichloromethane and methanol (1:1) into a round bottom flask, add furfural 1.8g (20mmol) and benzylamine 2.5g (24mmol) successively under stirring, react at 45°C for 1h, then cool down to 25°C, Add 500 mg NaBH three times in 45 minutes 4 , After 3 hours of reaction, the temperature was raised to 45°C, and the reaction was stopped after 4 hours, and the solvent was evaporated to dryness under reduced pressure. Add 20ml of dichloromethane again to dissolve, wash with saturated saline and deionized water three times, evaporate the solvent under reduced pressure, and separate by column chromatography to obtain 3.4g of intermediate 2 with a yield of 79.0%.
[0028] 2. Preparation of Intermediate 4
[0029]
[0030] In a round bottom flask, add 0.98g (10mmol) of maleic acid glycoside and dissolve it in 30ml of ether solvent. After it ...
Embodiment 2
[0037] Embodiment 2: the preparation of compound b
[0038] Except for using n-butylamine instead of benzylamine, the synthesis method was the same as that of compound a in Example 1, and the total yield was 52.3%. The structural formula is as follows:
[0039]
[0040] ESI MS:383.7[M+H] +1 , 1 H-NMR(300MHz,DMSO)δ8.21-8.14(m,1H),7.93-7.80(m,2H),6.97-6.74(m,3H),4.74(s,2H),4.17-4.15(m, 1H), 3.76(s, 3H), 3.53(s, 3H), 1.91-1.67(m, 2H), 1.33-1.29(m, 6H), 0.85(s, 3H).
Embodiment 3
[0041] Embodiment 3: the preparation of compound c
[0042] Except that n-propylamine was used instead of benzylamine, the synthesis method was the same as that of compound a in Example 1, and the total yield was 48.9%. The structural formula is as follows:
[0043]
[0044] ESI MS:369.1[M+H] +1 , 1 H-NMR(300MHz,DMSO)δ8.21-8.14(m,1H),7.93-7.80(m,2H),6.97-6.74(m,3H),4.74(s,2H),4.17-4.15(m, 1H), 3.76(s, 3H), 3.52(s, 3H), 1.93-1.67(m, 2H), 1.31-1.27(m, 4H), 0.86(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com