A kind of preparation method of inner salt of acridine
An acridine and compound technology, which is applied in the synthesis of chemiluminescent agents and the preparation of acridine inner salts, can solve the problems of low atomic economic efficiency, high synthesis difficulty, low combined yield, etc. The effect of improving the expandability and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
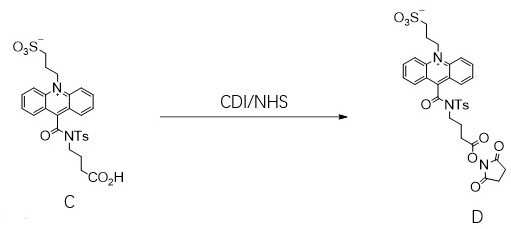
[0026] See attached figure 1 The shown chemical reaction formula is prepared from 10-(3-sulfopropyl)-N-p-toluenesulfonyl-N-(3-carboxypropyl)acridine-9-carboxamide, i.e. formula C compound 9-[ [[4-[(2,5-dioxo-1-pyrrolidinyl)oxy]-4-oxobutyl][(4-methylphenyl)sulfonyl]amino]carbonyl]-10- (3-sulfopropyl)-acridine inner salt, that is, the compound of formula D: after dissolving 200 mg of compound C in acetonitrile, the reaction solution was not dissolved, and 150 mg of N,N'-carbonyldiimidazole was added, N 2 After ventilation, wrap it in tinfoil paper, activate it at room temperature for 2 hours, and gradually dissolve the reaction solution, then add 194 mg of N-hydroxysuccinimide, react at room temperature for 6 hours, and a yellow solid precipitates out. HPLC tracking shows that the reaction of the raw materials is complete. Add 6 tert-butyl methyl ether twice the volume of acetonitrile was stirred and crystallized, suction filtered, the filter cake was washed with a small amount...
Embodiment 2
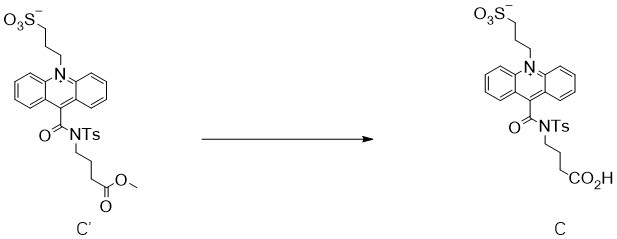
[0031] See attached figure 2 The reaction formula for the preparation of the shown C compound from its ester compound C': take 750 mg of the formula C' compound and add it to the HCl solution with a concentration of 3N, wrap it in tin foil, and use N 2 Pump and change air several times, react at 80°C for 16h, a yellow solid precipitates out of the reaction liquid, after the reaction is complete, cool down to room temperature, add NaHCO 3 Adjust the pH of the solid to alkaline, dissolve the yellow solid, extract it with dichloromethane three times, collect the water phase, spin off the remaining organic solvent in the water phase under reduced pressure, use HCl to adjust the pH of the water phase to 1, and the yellow solid precipitates , filtered with suction, washed the filter cake with water, and dried at 50°C. Obtained 652 mg of yellow solid, namely the compound of formula C, HPLC > 99%, yield: 89%.
Embodiment 3
[0033] See attached image 3 The reaction formula for preparing the compound of formula C' from the compound of formula B as shown: take 0.99 gram of compound of formula B, add 0.77 gram of 1,3-propane sultone, add 5 mL of N-methylpyrrolidone, and use N 2 Air exchange several times, shading, heating reaction in an oil bath at 125°C, reaction for 26 hours, stop heating, cool to room temperature, and direct column chromatography to obtain 1.11g of yellow powder, which is the compound of formula C', HPLC: 91.5%, yield: 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com