Serum-free culture medium for stem cells and application of serum-free culture medium
A serum-free medium and stem cell technology, applied in the field of stem cell serum-free medium, can solve the problems of complex formula, affecting medical efficacy, limiting popularization, etc., and achieve the effect of clear chemical composition, excellent proliferation effect and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
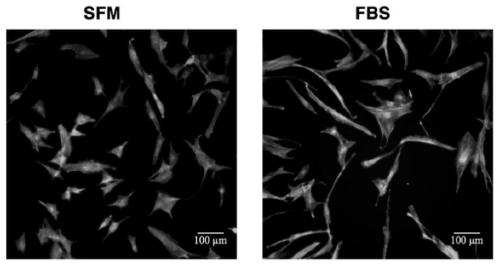
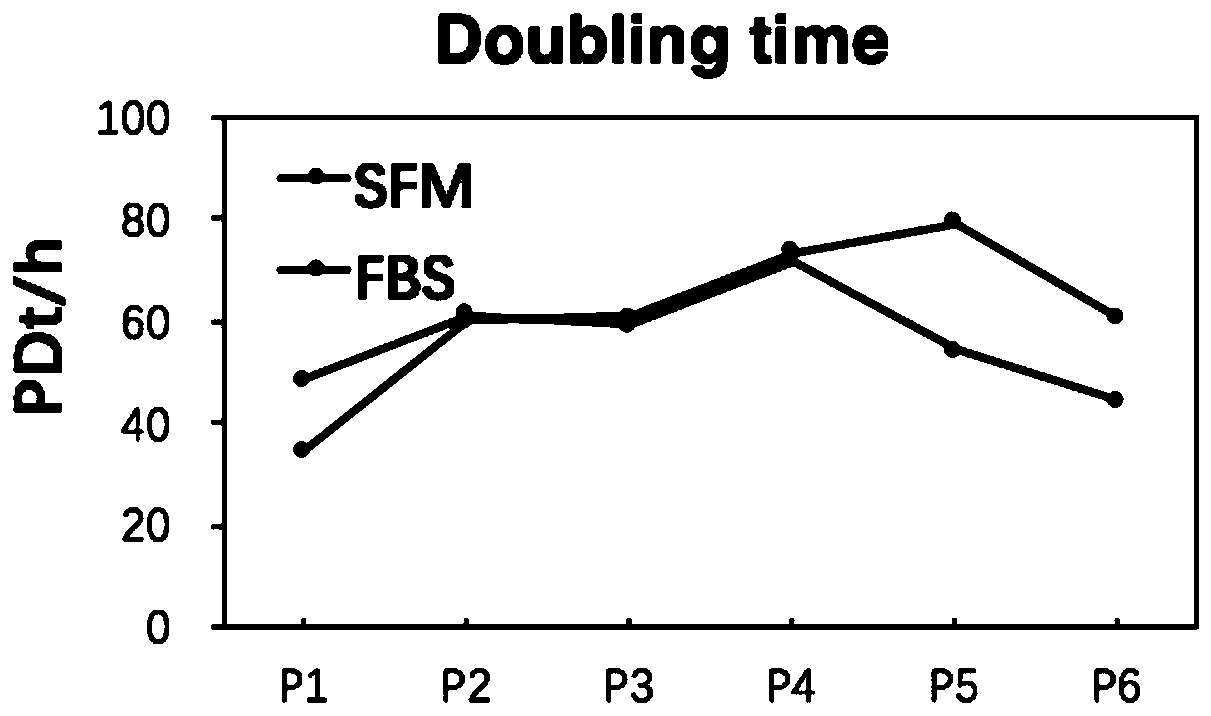
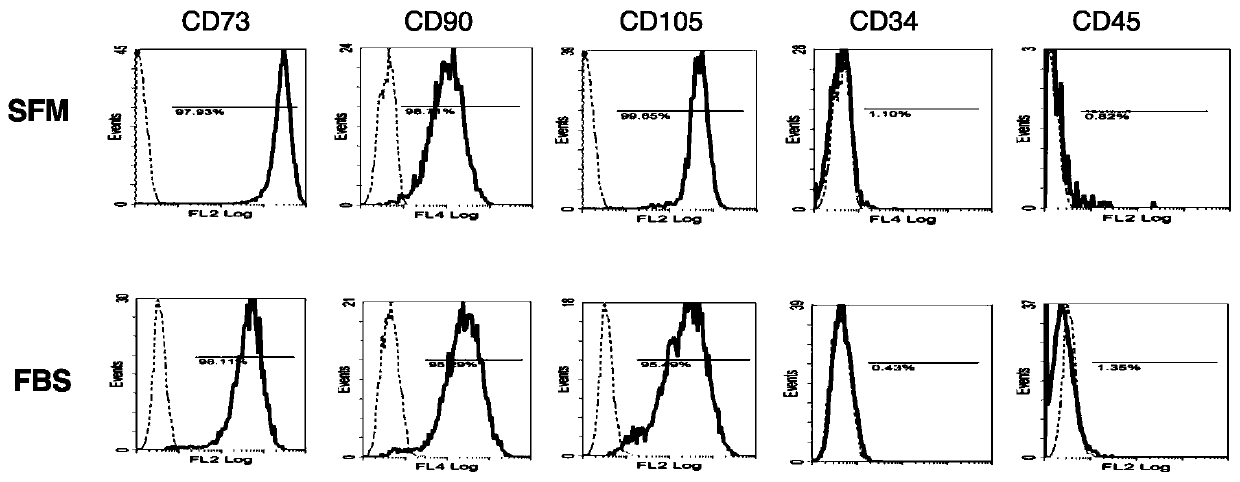
[0116] In this example, two sets of culture media were prepared, namely serum-free medium (SFM) and control medium (FBS). The culture medium of the control group is DMEM low-glucose medium added with 10% fetal bovine serum. The cultured cells are human adipose stem cells.
[0117] The following are detailed experimental and detection steps:
[0118] 1. The configuration of the culture medium (no need to coat when configuring)
[0119] The serum-free medium components of the present embodiment are as follows: add the following components in the DMEM low-sugar basal medium (GIBCO) of every 500mL, and make the concentration of the added components in the serum-free medium be:
[0120] Recombinant Human Serum Albumin 20mg / mL
[0121] Recombinant human PDGF-AA 20ng / mL
[0122] Recombinant human PDGF-BB 20ng / mL
[0123] Recombinant human bFGF 5ng / mL
[0124] Recombinant human TGF-β1 5ng / mL
[0125] Recombinant Human EGF 20ng / mL
[0126] Recombinant IGF 20ng / mL
[0127] Reco...
Embodiment 2
[0169] In this example, two sets of culture media were prepared, namely serum-free medium (SFM) and control medium (FBS). The culture medium of the control group is DMEM low-glucose medium added with 10% fetal bovine serum. The cultured cells are human adipose stem cells.
[0170] The following are detailed experimental and detection steps:
[0171] 1. Configuration of culture medium
[0172] Serum-free medium: add additional components in the DMEM low-sugar medium (GIBCO) of every 500mL, and make the concentration of the additional components in this serum-free medium be:
[0173] The hydrolysis rate of polyvinyl alcohol is 75-90%
[0174] PVC 10mg / mL
[0175] PDGF synthetic peptide 20ng / mL
[0176] Recombinant human bFGF 5ng / mL
[0177] Recombinant human TGF-β1 5ng / mL
[0178] Recombinant Human EGF 20ng / mL
[0179] Recombinant IGF 20ng / mL
[0180] Fibronectin FN synthetic peptide 5 μg / mL
[0181] Heparin 5μg / mL
[0182] Lipid Concentrate 0.1% (v / v)
[0183] Recom...
Embodiment 3
[0199] The cells are human umbilical cord stem cells, and the cell culture method is the same.
[0200] Serum-free medium: add additional components in the DMEM / F12 (GIBCO) of every 500mL, and make the concentration of the additional components in this serum-free medium be:
[0201] The hydrolysis rate of polyvinyl alcohol is 75-90%
[0202] PVC 10mg / mL
[0203] Recombinant human PDGF-AA 20ng / mL
[0204] Recombinant human FGF 5ng / mL
[0205] Recombinant human TGF-β1 5ng / mL
[0206] Recombinant Human EGF 20ng / mL
[0207] Recombinant human IGF 20ng / mL
[0208] Recombinant human fibronectin FN 5 μg / mL
[0209] Heparin 5μg / mL
[0210] Lipid Concentrate 0.1% (v / v)
[0211] Recombinant insulin 2 μg / mL
[0212] Transferrin 1 μg / mL
[0213] Sodium selenite 1ng / mL
[0214] Galactose 20mM
[0215] L-Glutamine 292mg / L
[0216] Putrescine 50μM
[0217] Progesterone 20nM
[0218] Hydrocortisone 100nM
[0219] Vitamin C 200μM
[0220] Vitamin A 50μM
[0221] Sodium bicarbona...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com