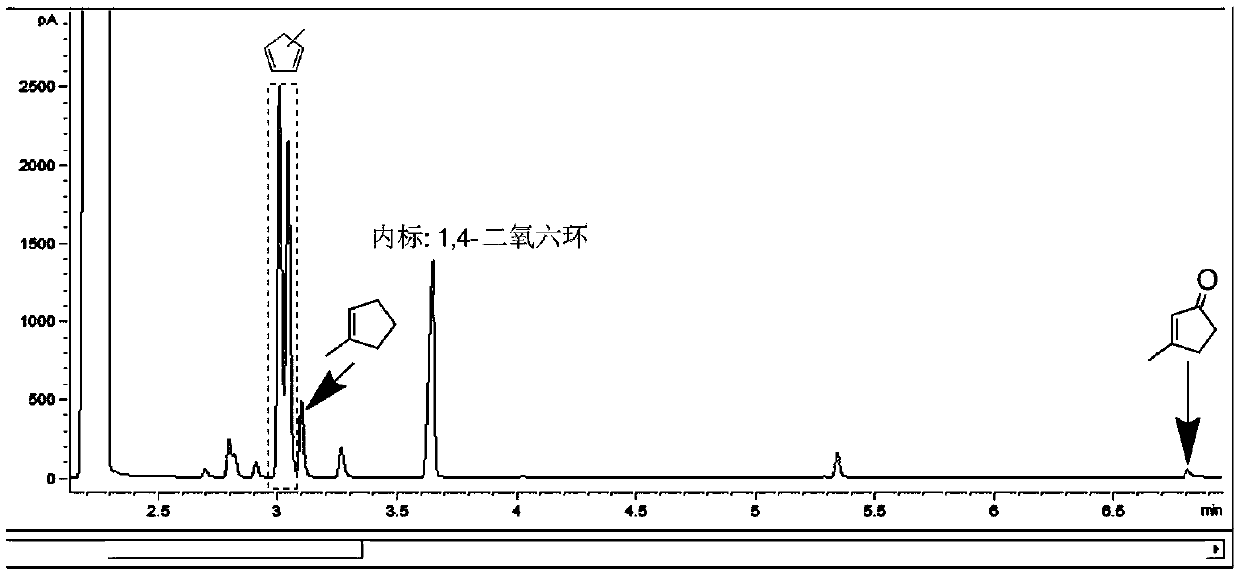
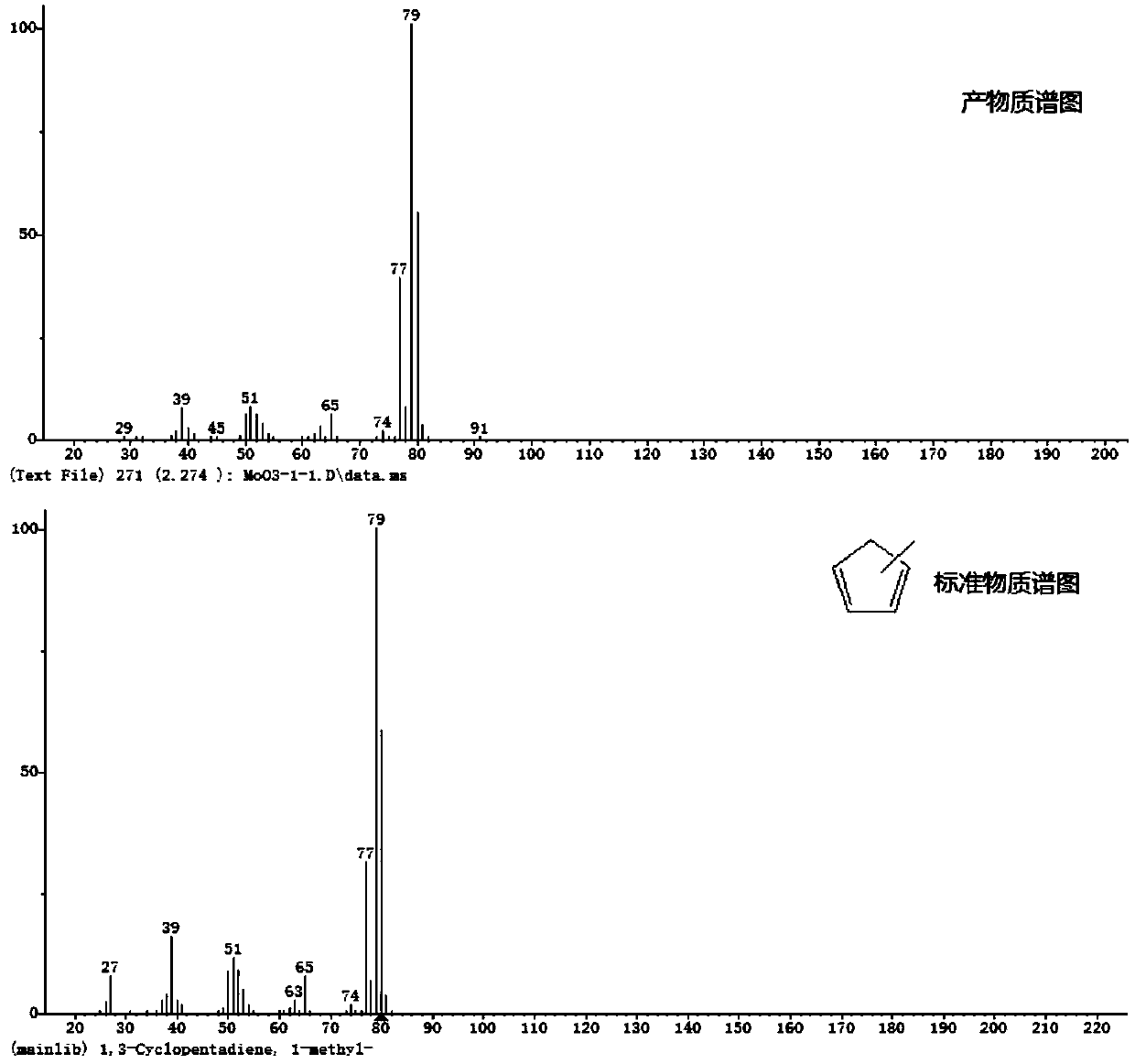
Method for synthesizing methyl cyclopentadiene from 3-methyl-2-cyclopentene-1-one
A technology of methylcyclopentadiene and cyclopentene, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., to achieve the effects of mild reaction conditions, simple catalyst preparation, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Supported metal oxide A / X type catalyst Co 3 O 4 / SiO 2 Preparation: Weigh 3 grams of SiO pretreated at 250℃ for 15h 2 , Immerse it in an equal volume of an aqueous solution containing 2.90 g of cobalt nitrate, immerse it for 12 hours and then dry it at 60°C for 48 hours, then calcinate it at 500°C for 4 hours, and then press into a tablet. Co prepared 3 O 4 / SiO 2 Metal oxide Co in catalyst 3 O 4 The content is 21Wt%, the carrier SiO 2 The content is 79Wt%.
[0022] The above Co 3 O 4 / SiO 2 One gram of catalyst is packed in a fixed-bed continuous reactor, and then the hydrogen pressure is 0.2MPa and the hydrogen space velocity is 500h. -1 The reduction temperature is 450℃ for 1 hour, and then the bed temperature of the fixed-bed continuous reactor is kept at 450℃, and the reaction pressure is controlled to 0.02MPa. The space-time of 3-methyl-2-cyclopenten-1-one Speed is 0.5h -1 , The molar ratio of hydrogen to 3-methyl-2-cyclopenten-1-one is 50:1, the conversion rat...
Embodiment 2
[0024] (1) Supported metal oxide A / X type catalyst Fe 3 O 4 / ZrO 2 Preparation: After preparing 100 grams of 5 grams of ferric nitrate aqueous solution, it is divided into two parts B and C with equal mass, and 10 grams of carrier ZrO is added to B 2 , Add 15 grams of urea to C, slowly add C to B in a water bath at 80°C, keep it at 90°C for 10 hours and then dry at 120°C for 48 hours, then calcinate at 600°C for 4 hours, and then press into tablets. The metal oxide component Fe in the prepared catalyst 3 O 4 The content is 9Wt%, carrier ZrO 2 The content is 91Wt%.
[0025] The above Fe 3 O 4 / ZrO 2 One gram of catalyst is packed in a fixed-bed continuous reactor, and then the hydrogen pressure is 0.5MPa and the hydrogen space velocity is 2000h. -1 、Reduction at 400℃ for 3 hours, then keep the bed temperature of the fixed-bed continuous reactor at 400℃, control the reaction pressure to 0.3MPa, and the hourly space velocity of 3-methyl-2-cyclopenten-1-one 2h -1 , The molar ratio of ...
Embodiment 3
[0027] (1) Supported metal oxide A / X type catalyst WO 3 / CeO 2 Preparation: Weigh 3 grams of CeO pretreated at 600°C for 2 hours 2 , Immerse the same volume in an aqueous solution containing 4 g of sodium tungstate, immerse for 5 hours and then dry at 120°C for 12 hours, then calcinate at 700°C for 1 hour, and then press into tablets. Prepared WO 3 / CeO 2 Metal oxide component WO in catalyst 3 The content is 49Wt%, the carrier CeO 2 The content is 51Wt%.
[0028] Put the above WO 3 / CeO 2 One gram of catalyst is packed in a fixed-bed continuous reactor, and then the hydrogen pressure is 0.8MPa and the hydrogen space velocity is 4000h -1 、Reduction at 500℃ for 1 hour, then control the reaction temperature at 500℃, the reaction pressure is 0.5MPa, and the hourly space velocity of 3-methyl-2-cyclopenten-1-one is 4h -1 , The molar ratio of hydrogen to 3-methyl-2-cyclopenten-1-one is 200:1, the conversion rate of 3-methyl-2-cyclopenten-1-one is 100%, and the methyl ring The selectivity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com