Compound Disalbaspidin PB and application of compound Disalbaspidin PB in antibiosis
A compound and anti-bacterial technology, applied in the field of medicine, can solve the problem of few active ingredients, achieve clear structure, good application prospects, and promote effective application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
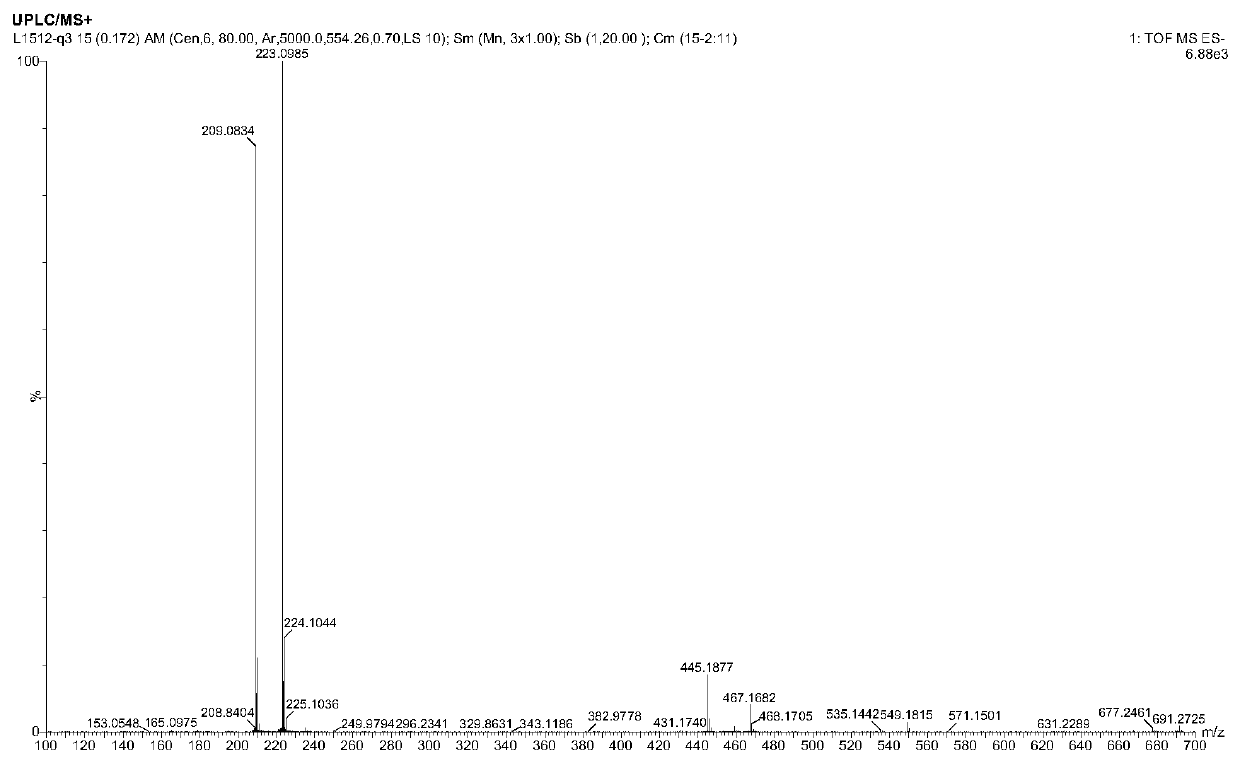
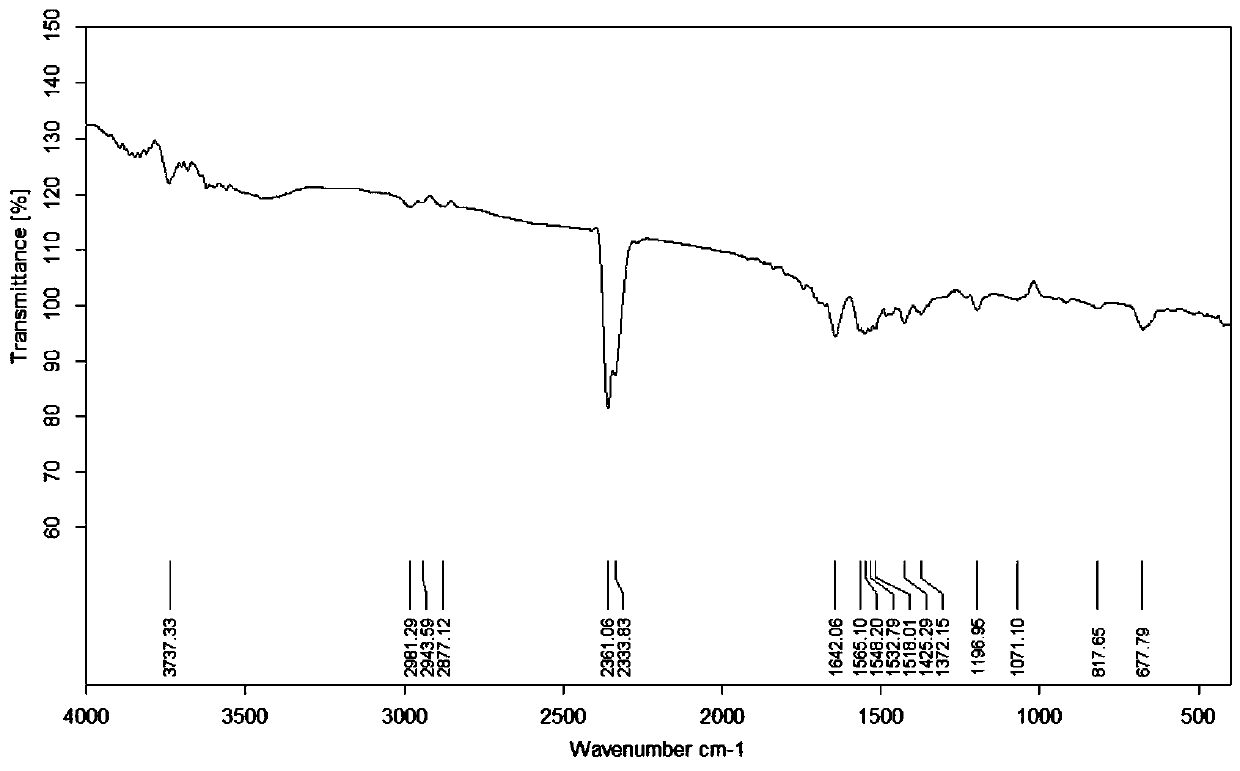
[0050] Example 1 Extraction and Identification of Disalbaspidin PB
[0051] 1. Extraction method
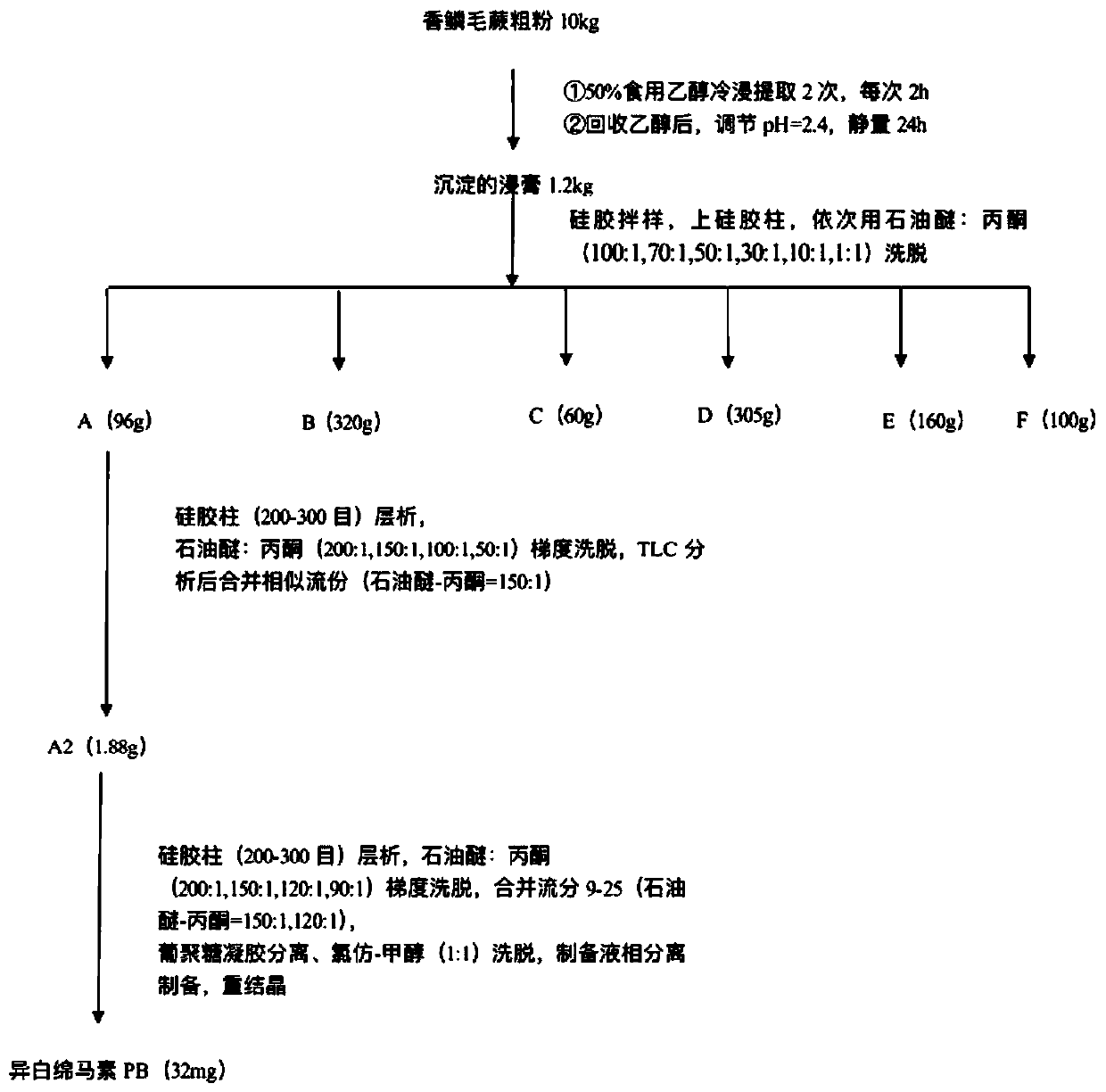
[0052] The method for extracting compound Disalbaspidin PB from D. figure 1 As shown, it specifically includes the following steps:
[0053] S1. Extraction of extract: 10kg of dry powder of Trichophyllum chrysalis is refluxed and extracted twice at room temperature with 50% ethanol by volume fraction of 10 times, the time is 2 hours respectively, the pH is adjusted to 2.4 with hydrochloric acid, and the medicine soaked in ethanol The liquid is concentrated and extracted to obtain 1.2kg of extract;
[0054] S2. After dissolving the precipitated extract sample obtained in S1, mix the sample with silica gel, perform preliminary separation on the silica gel column, and use different proportions of petroleum ether: acetone (100:1, 70:1, 50:1, 30:1, 10:1,1:1), to obtain the corresponding components A (96g), B (320g), C (60g), D (305g), E (160g), F (100g);
[0055] S3. Take componen...
Embodiment 2
[0071] Example 2 The inhibitory effect of Disalbaspidin PB on fungi
[0072] 1. Experimental method
[0073] Gently scrape the colonies on the surface of the SDA medium with an inoculation loop, grind the mycelium with a sterile grinder, suspend the dermatophytes in sterile saline, adjust the turbidity to 0.5 McFarland turbidity, and count the spores with a hemocytometer number and number of short hyphae. Make the bacterial concentration 1×10 3 CFU / mL to 3×10 3 CFU / mL. That is, the 0.5 McFarland turbidity bacteria solution was diluted 1000 times with RPMI-1640 liquid medium, and counted by the hemocytometer to obtain the inoculum solution. Use RPMI-1640 liquid medium to dilute the stock solution (20μg / mL) of Trichofernium monomer in the first column of 96-well plate, use the culture medium to carry out horizontal 2-fold dilution to the 10th column, add 100μL RPMI- 1640 liquid medium, add 200 μL RPMI-1640 liquid medium to column 12. Then add 100 μL of the inoculum solutio...
Embodiment 3
[0081] Example 3 The inhibitory effect of Disalbaspidin PB on bacteria
[0082] 1. Experimental method
[0083] Test method: The microdilution method is used, which is often used to determine the antibacterial activity of sensitive drugs or new drugs against human pathogenic bacteria. The operation method is simple and can be used for a large number of antibacterial tests. Using nutrient broth medium, the activated clinical pathogenic strains were inoculated into the medium to prepare a bacterial liquid, and the drug to be tested was dissolved in DMSO to prepare 64mg / mL. Dilute the drug to be tested line by line in a 96-well plate by two-fold decreasing dilution method; then add the diluted bacterial solution, and set aside two wells as a control, one well only adds medium, and the other well only Add bacterial solution, shake and mix evenly after dilution, then place the 96-well plate in a 35°C constant temperature incubator and incubate for 18-24 hours. The minimum inhibito...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com