Application of oxyberberine in preparation of medicament for treating ulcerative colitis
A technology for ulcerative colitis and berberine, applied in the field of biomedicine, can solve problems such as curative effect and safety limitations, achieve anti-arrhythmic efficacy, good application prospects, and improve the effect of general symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
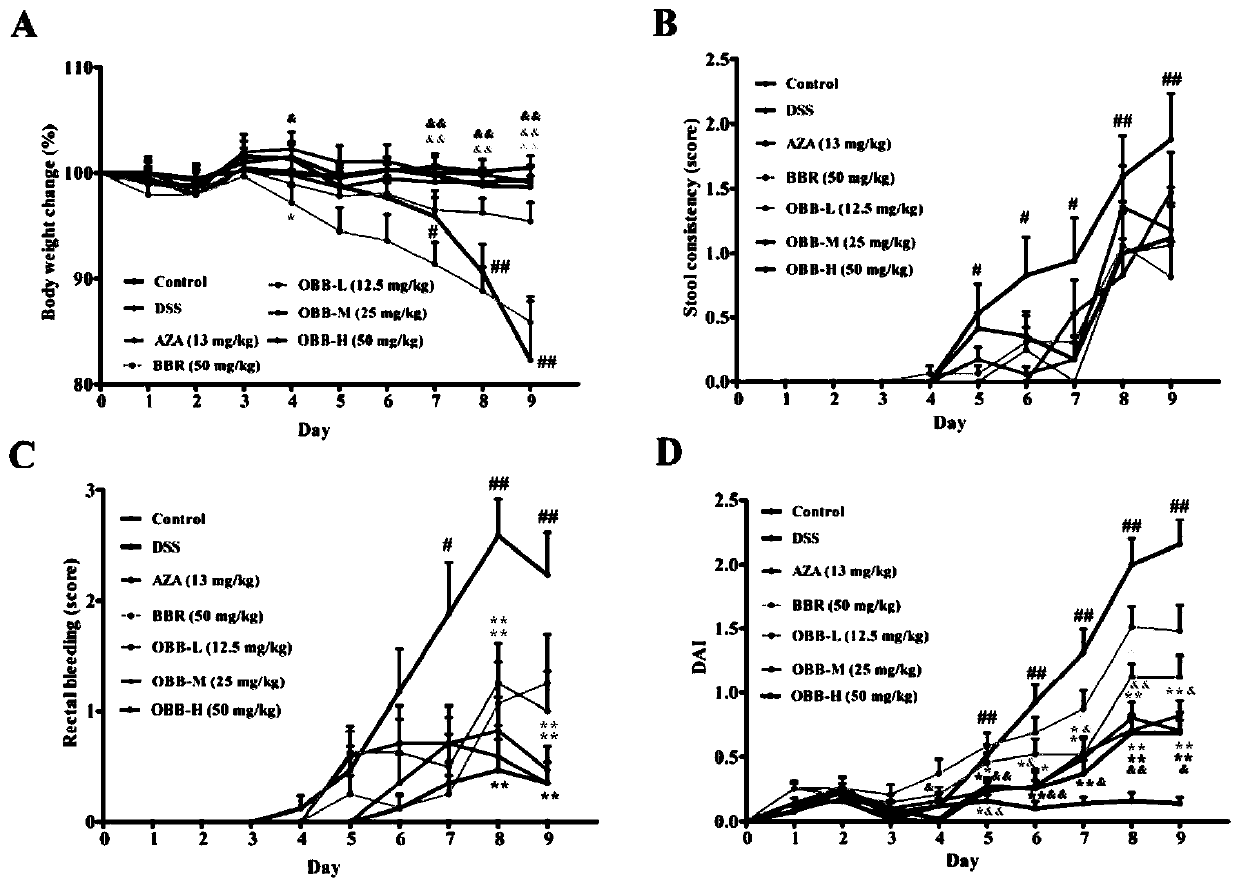
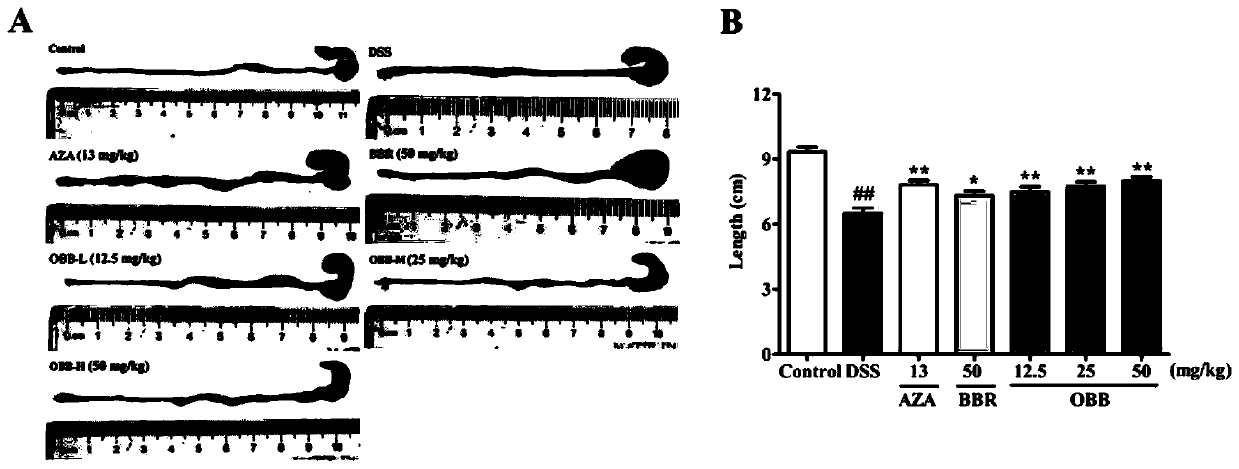
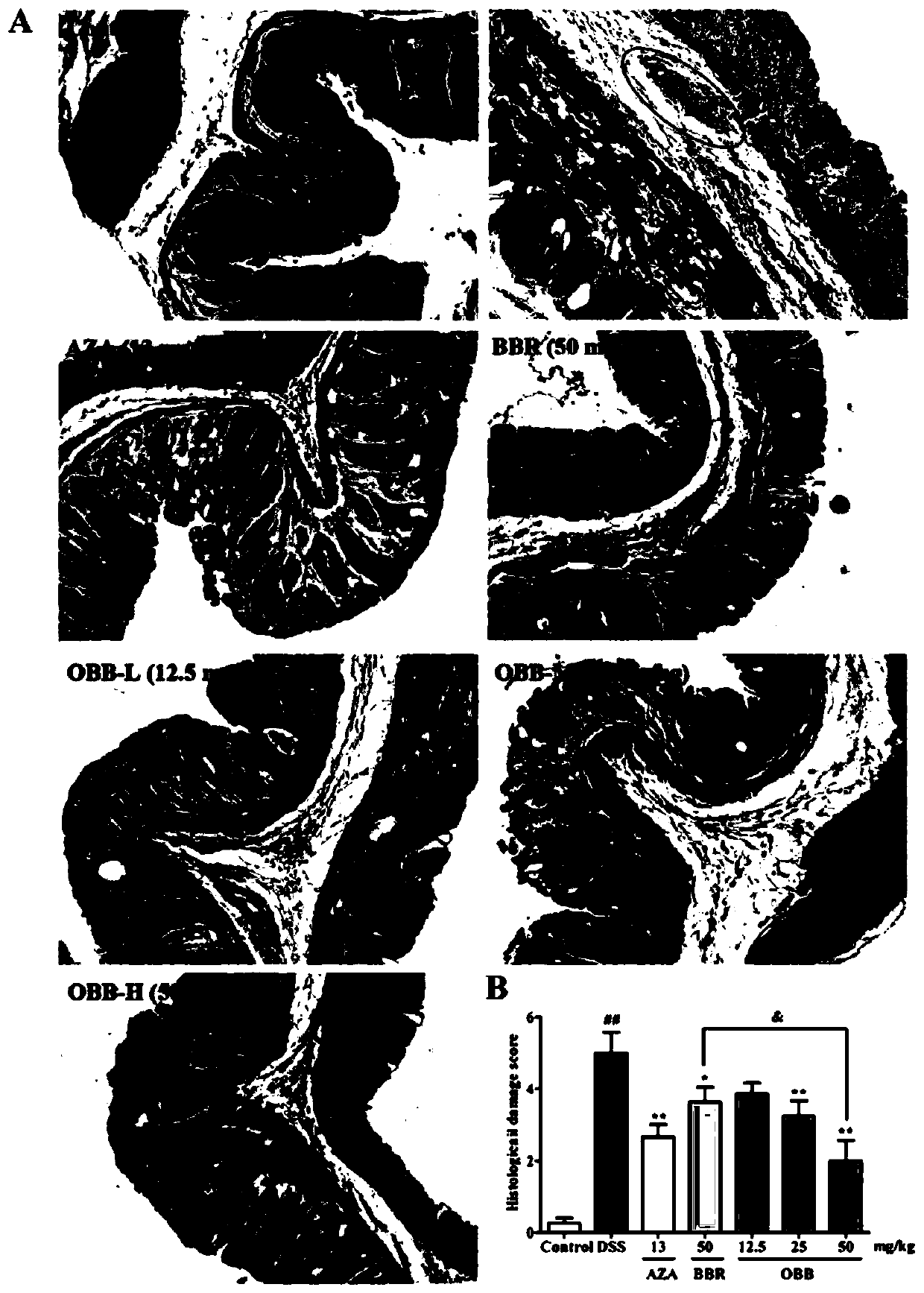
[0033] The effect of embodiment 1 oxidized berberine on ulcerative colitis
[0034] 1 Experimental materials
[0035] 1.1 Experimental drugs
[0036] Berberine hydrochloride (BBR) was purchased from Chengdu Zhibiao Pure Biotechnology Co., Ltd.; oxidized berberine (OBB, self-made) (purity ≥ 98%); azathioprine (AZA) was purchased from Aspen Pharmacare Australia Pty Ltd. (Leonards, New South Wales, Australia).
[0037] 1.2 Experimental reagents
[0038] Dextran sodium sulfate (DSS, MW: 36000-50000) was purchased from MP Biomedicals, USA; the H&E staining kit was purchased from Wuhan Boster Biological Engineering Co., Ltd.; myeloperoxidase (MPO) kit was purchased from Nanjing Jiancheng Institute of Bioengineering; mouse ELISA kits: TNF-α, IL-6, IL-1β, IL-10, IL-17 and IFN-γ, purchased from Beijing Chenglin Biology; mouse IgA, IgM and IgG Elisa kits were purchased from Xinbosheng Biotechnology Co., Ltd. Antibodies JAM-A, Claudin-1, Occludin, ZO-1, ZO-2, β-actin and horseradish ...
Embodiment 2
[0088] Embodiment 2 treats the oxidized berberine medicament (tablet) of ulcerative colitis
[0089] Take 500g of oxidized berberine, add 480g of lactose and 754g of starch, mix evenly, use 7% starch slurry 350g as a binder, wet granulate, dry, add 16g of magnesium stearate, mix evenly, and press to make each tablet contain oxidized berberine. 10,000 tablets of berberine 50mg, each with a net weight of 0.21g.
[0090] Orally, for the treatment of ulcerative colitis.
[0091] Symptoms: Abdominal pain, diarrhea, blood in the stool, weight loss, etc.
Embodiment 3
[0092] Embodiment 3 treats the oxidized berberine medicament (capsule) of ulcerative colitis
[0093] Take 500g of oxidized berberine, add 980g of lactose, 1254g of starch and mix evenly, use 7% starch slurry 350g as a binder, wet granulate, dry, add 16g of magnesium stearate, mix evenly, fill to No. 1 capsule 10,000 capsules, each capsule contains 50mg of oxidized berberine, and the net weight of each capsule is 0.31g.
[0094] Orally, for the treatment of ulcerative colitis.
[0095] Symptoms: Abdominal pain, diarrhea, blood in the stool, weight loss, etc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com