Synthetic process of dorzolamide Hydrochloride intermediate
A technology of dorzolamide hydrochloride and synthesis process, applied in the directions of organic chemistry, organic chemistry methods, etc., can solve the problems of harsh equipment performance and material requirements, unfriendly environment, difficult operation, etc., so as to reduce pollution and damage, and avoid operation hazards. Sex, damage reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
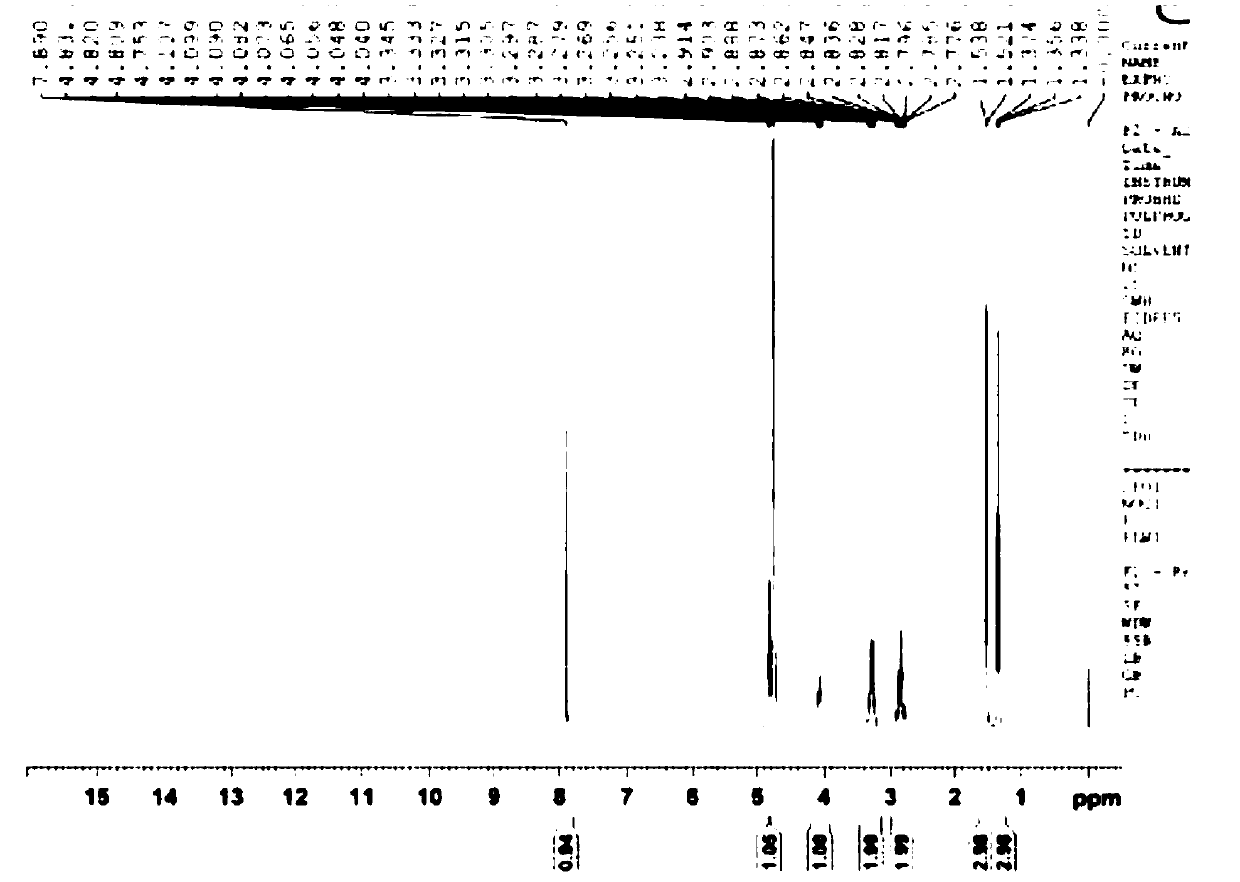
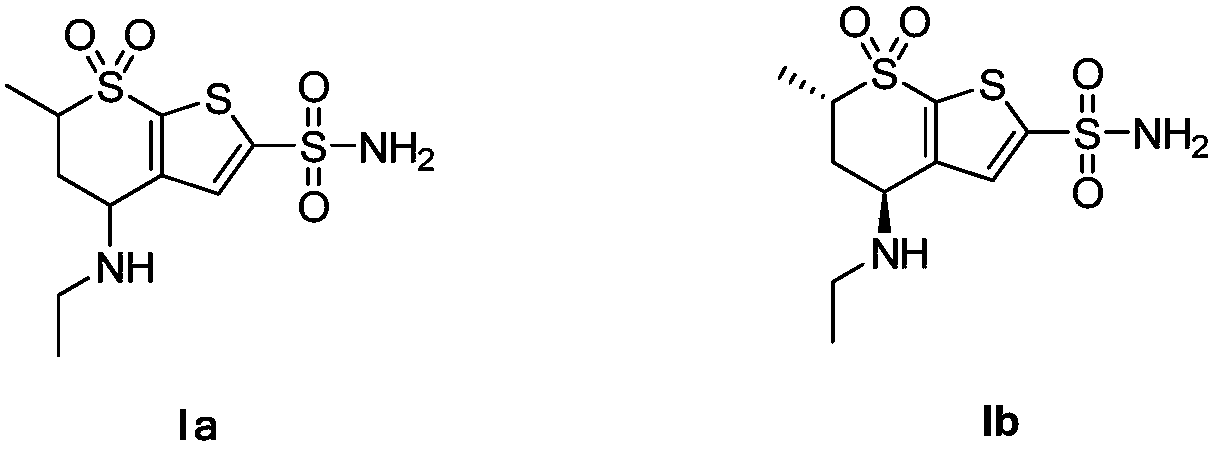
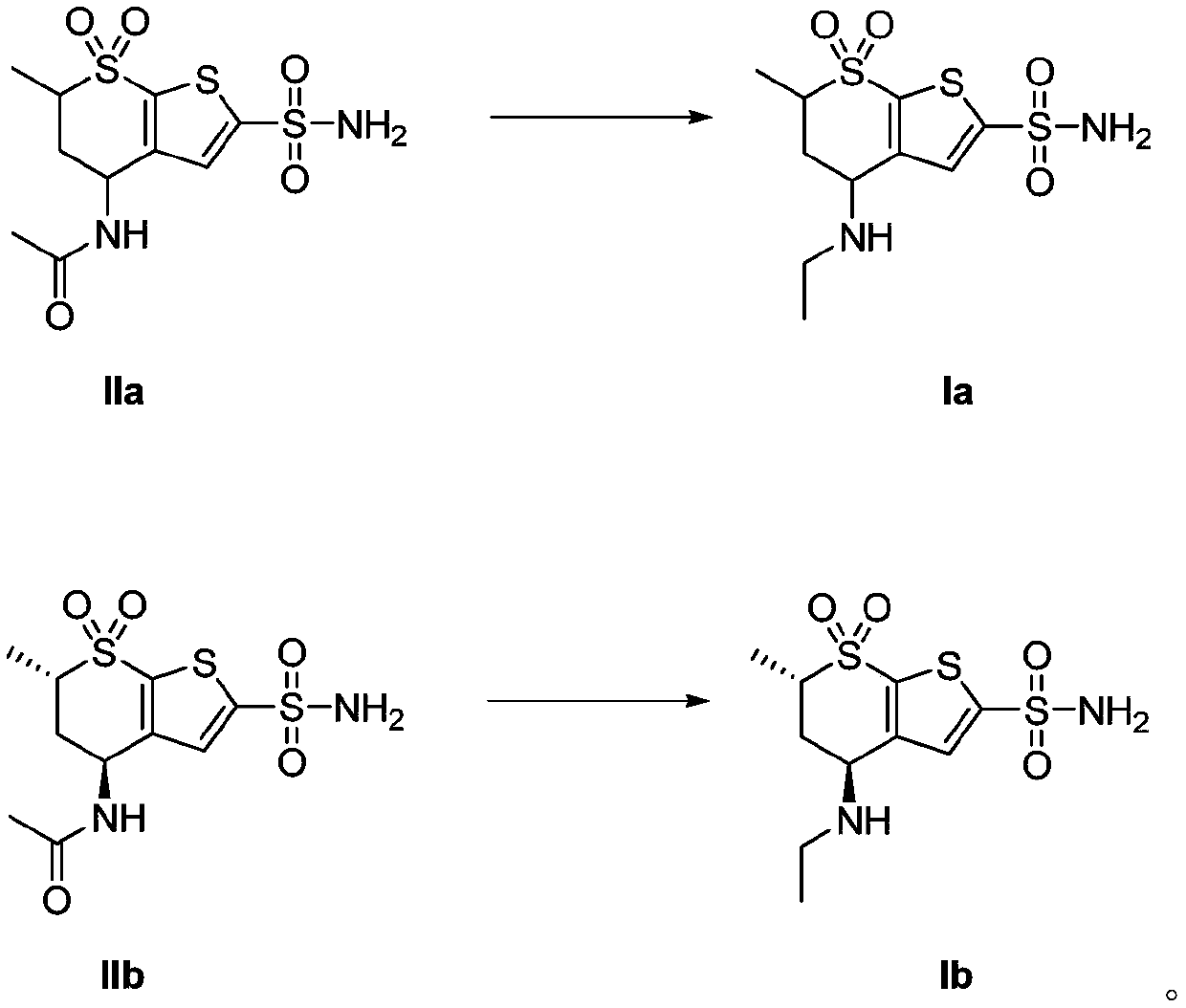
[0067] 20 g of starting compound IIa (4-acetamido-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-2-sulfonamide-7,7-dioxide ), 20g of zinc borohydride and 200ml of tetrahydrofuran were added to the reaction kettle, the temperature in the reaction kettle was heated to 70°C, and the reaction was stirred. After 2.5 hours of reaction, the reaction was tracked and monitored by TLC. After the complete reaction of the raw material compound IIa was detected, the When the temperature in the reaction kettle drops to 20°C, slowly add 300ml of 1M sulfuric acid into the reaction kettle dropwise. During the dropping process, control the temperature in the reaction kettle to 20°C. After reacting for 1h, the temperature in the reactor was lowered to room temperature, ethyl acetate was added for extraction twice, and 200ml of ethyl acetate was added for each extraction, and finally the organic phase obtained by extraction was concentrated to obtain 16.8g of white solid (Ia). 92%.
Embodiment 2
[0069] 20 g of starting compound IIa (4-acetamido-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-2-sulfonamide-7,7-dioxide ), 16g of zinc borohydride and 150ml of dioxane were added to the reactor, the temperature inside the reactor was heated to 80°C, and the reaction was stirred. After 3 hours of reaction, the reaction was tracked and monitored by TLC. After the complete reaction of the raw material compound IIa was detected , when the temperature in the reactor is lowered to 25°C, slowly drop 250ml of 1M sulfuric acid into the reactor, and control the temperature in the reactor to 20°C during the dropping process, and then raise the temperature in the reactor to 85°C after the addition is completed , after stirring and reacting for 2 hours, the temperature in the reactor was lowered to room temperature, ethyl acetate was added for extraction twice, and 200 ml of ethyl acetate was added for each extraction, and finally the organic phase obtained by extraction was concentrated...
Embodiment 3
[0071] 20 g of starting compound IIa (4-acetamido-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-2-sulfonamide-7,7-dioxide ), 12g of zinc borohydride and 100ml of acetic acid were added to the reactor, the temperature in the reactor was heated to 90°C, and the reaction was stirred. After 3 hours of reaction, TLC was used to track and monitor the reaction. After monitoring that the reaction of the raw material compound IIa was complete, the reaction When the temperature in the kettle drops to 30°C, slowly add 200ml of 1M sulfuric acid into the reaction kettle dropwise. During the dropping process, control the temperature in the reaction kettle to 30°C. After 2.5h, the temperature in the reactor was lowered to room temperature, ethyl acetate was added for extraction twice, and 200ml of ethyl acetate was added for each extraction, and finally the organic phase obtained by extraction was concentrated to obtain 16.5g of white solid (Ia). 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com