Method for preparing lithium hydroxide monohydrate from spodumene sulfuric acid leaching solution
A technology of lithium hydroxide monohydrate and leaching solution, which is applied to the improvement of process efficiency, diaphragms, cells, etc., can solve the problems of low utilization rate of lithium ore resources, inability to meet low-carbon environmental protection, waste of water resources, etc., to meet the requirements, The effect of reducing production costs, saving resources and energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
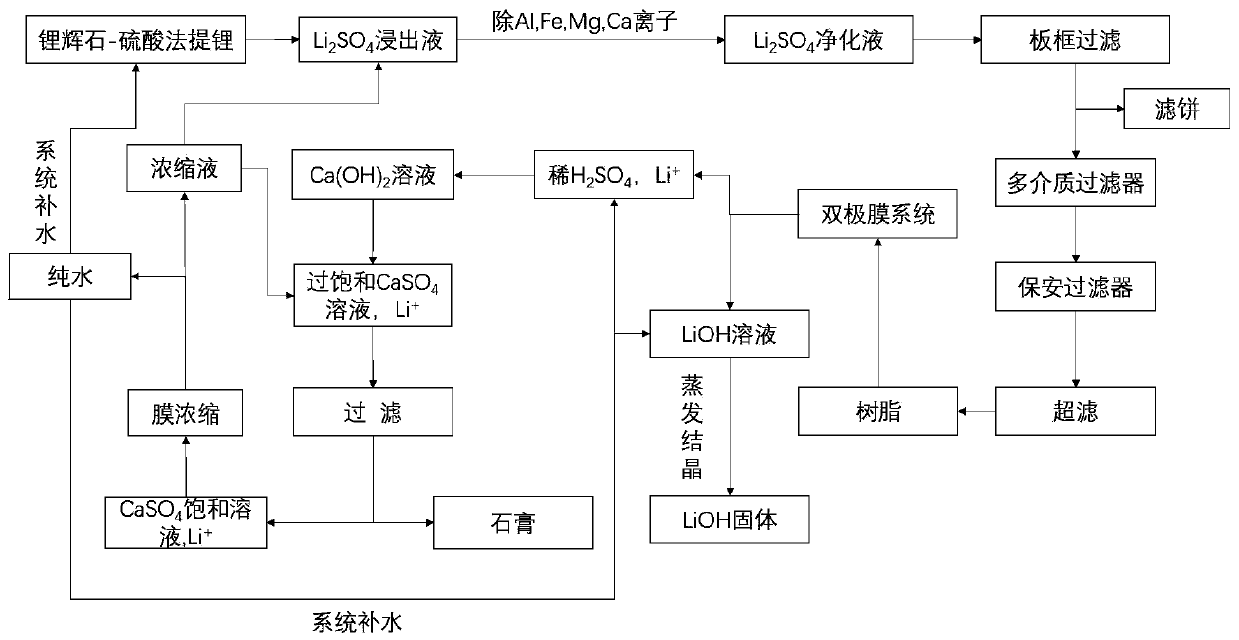
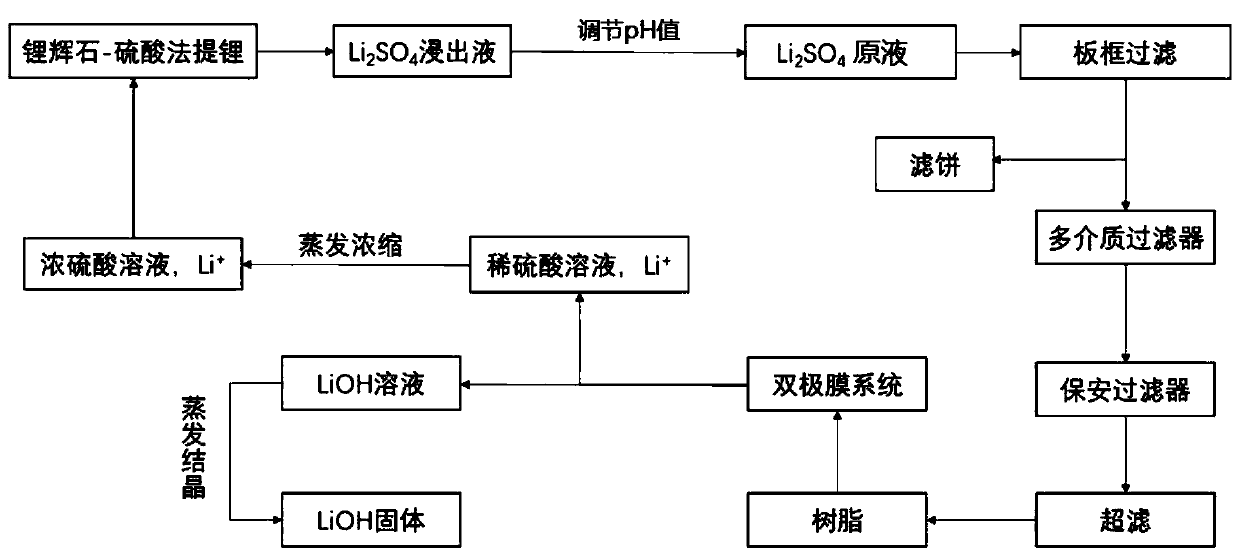
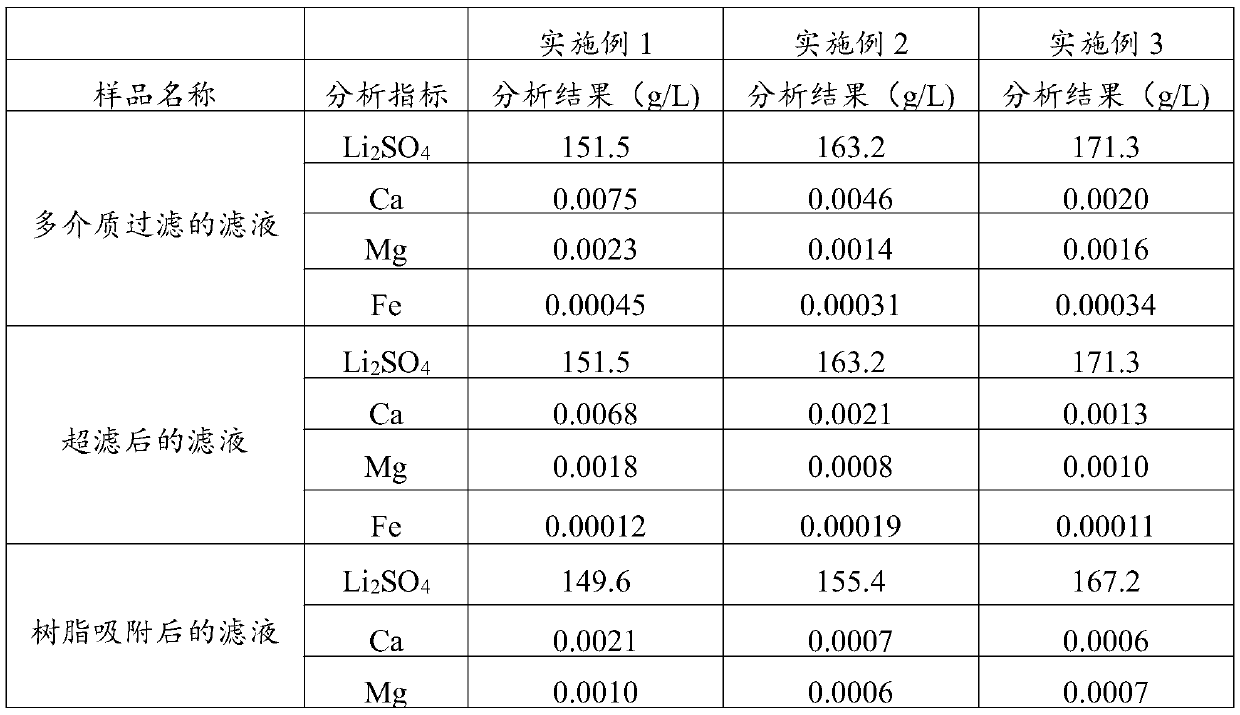
[0071] (1) After the spodumene is sulphated, roasted and leached, a lithium sulfate leaching solution with a mass concentration of 15.15% is obtained. The lithium sulfate leaching solution is sequentially passed through lithium hydroxide to adjust the pH value to 8, and plate and frame filtration (the pressure of the plate and frame filtration is 1.5 MPa, the pore size of the filter frame is 4μm), multi-media filtration (activated carbon, quartz sand and porous ceramics, the upper layer is activated carbon layer, the middle layer is quartz sand layer, and the lower layer is porous ceramic layer; the particle size of activated carbon is 0.6~1mm, stacked The density is 0.5t / m 3 ; The particle size of the quartz sand is 1.2 ~ 2.5mm, and the bulk density is 1.7t / m 3 The particle size of the porous ceramic is 2~4mm, and the bulk density is 2.0t / m 3 ), security filtration (the pore size of the security filtration membrane is 3μm), ultrafiltration (the pore size is 0.04μm) and the adsor...
Embodiment 2
[0076] (1) After the spodumene is sulphated, roasted and leached, a lithium sulfate leaching solution with a mass concentration of 16.32% is obtained. The lithium sulfate leaching solution is sequentially passed through lithium hydroxide to adjust the pH value to 10, and plate and frame filtration (the pressure of the plate and frame filtration is 1.6 MPa, the pore size of the filter frame is 5μm), multi-media filtration (activated carbon, quartz sand and porous ceramics, the upper layer is activated carbon layer, the middle layer is quartz sand layer, and the lower layer is porous ceramic layer; the particle size of activated carbon is 0.6~1mm, stacked The density is 0.45t / m 3 ; The particle size of the quartz sand is 1.2 ~ 2.5mm, and the bulk density is 1.6t / m 3 The particle size of the porous ceramic is 2~4mm, and the bulk density is 1.9t / m 3 ), security filtration (the pore size of the security filtration membrane is 2μm), ultrafiltration (the pore size is 0.05μm) and polysty...
Embodiment 3
[0081] (1) After the spodumene is sulphated, roasted and leached, a lithium sulfate leaching solution with a mass concentration of 17.13% is obtained. The lithium sulfate leaching solution is successively passed through lithium hydroxide to adjust the pH to 13, and plate and frame filtration (the pressure of the plate and frame filtration is 2.5MPa The pore size of the filter frame is 8μm), multi-media filtration (activated carbon, quartz sand and porous ceramics, the upper layer is activated carbon layer, the middle layer is quartz sand layer, and the lower layer is porous ceramic layer; the particle size of activated carbon is 0.6~1mm, and the bulk density 0.55t / m 3 ; The particle size of the quartz sand is 1.2 ~ 2.5mm, and the bulk density is 1.75t / m 3 The particle size of the porous ceramic is 2~4mm, and the bulk density is 2.1t / m 3 ), security filtration (the pore size of the security filtration membrane is 4μm), ultrafiltration (the pore size is 0.01μm) and polyacrylic resi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com