Water-soluble compounds with aggregation-induced emission characteristics
A technique for aggregation-induced luminescence and fluorescent compounds, applied in the field of fluorescent compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Synthesis of TTVP
[0101] 5-(4-(diphenylamino)phenyl)thiophene-2-carbaldehyde (71mg, 0.2mmol) and 1-(3-trimethylaminopropyl)-4-methylpyridinium dibromide (71mg, A solution of 0.2 mmol) was refluxed under nitrogen atmosphere in dry ethanol catalyzed by adding a few drops of piperidine overnight. After cooling to room temperature, the solvent was removed by distillation under reduced pressure. Separation by neutral alumina column and purification of the residue using DCM and methanol (98:2 v / v) as eluents afforded TTVP (98 mg, yield 71%) as a reddish-brown powder. 1 H NMR (400MHz, CD 3 OD), δ(ppm): 8.78(d, J=6.8Hz, 2H), 8.13-8.17(m, 3H), 7.58-7.60(m, 2H), 7.48(d, J=4.0Hz, 1H), 7.40(d, J=4.0Hz, 1H), 7.30-7.34(m, 4H), 7.02-7.12(m, 9H), 4.60(t, J=7.8Hz, 2H), 3.52-3.56(m, 2H) ,3.20(s,9H),2.51-2.59(m,2H). 13 C NMR (100MHz, CDCl 3 ),δ(ppm):155.99,150.81,150.19,148.72,145.26,140.33,136.72,135.83,130.81,128.12,126.40,125.16,125.04,124.90,123.80,121.71,64.04,58.04,54.13,2...
Embodiment 2
[0103] photophysical properties
[0104] TTVP has good water solubility. TTVP benefits from the hydrophilic nature of its positively charged amine and pyridinium salt, and the small size of the hydrophobic part. The aqueous solution of TTVP exhibits the maximum absorption band peak at 515nm, and the molar extinction coefficient is 33517M -1 cm -1 ( figure 2 ). The relatively long absorption wavelength can be attributed to its small HOMO-LOMO energy gap, which is caused by the strong electron donating-accepting interaction of the luminescent center ( Figures 3A to 3C ).
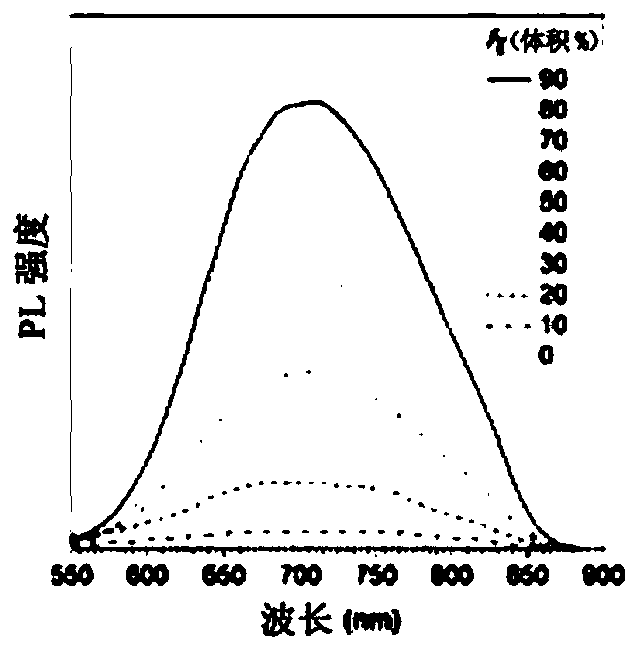
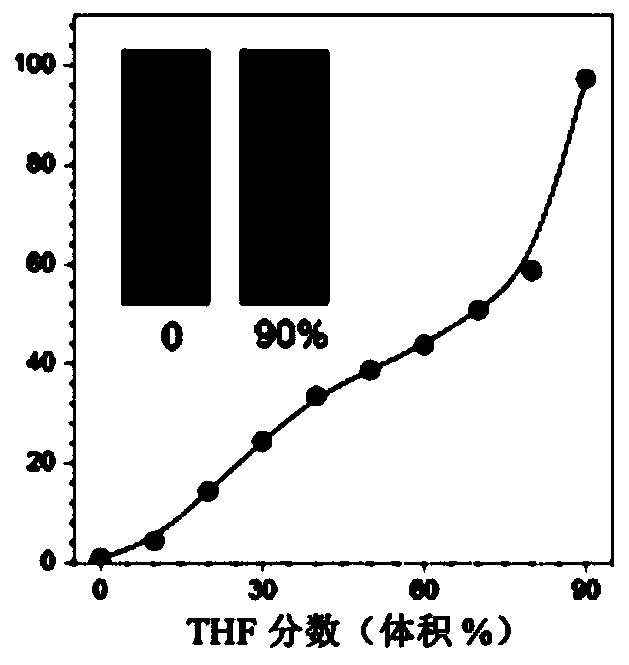
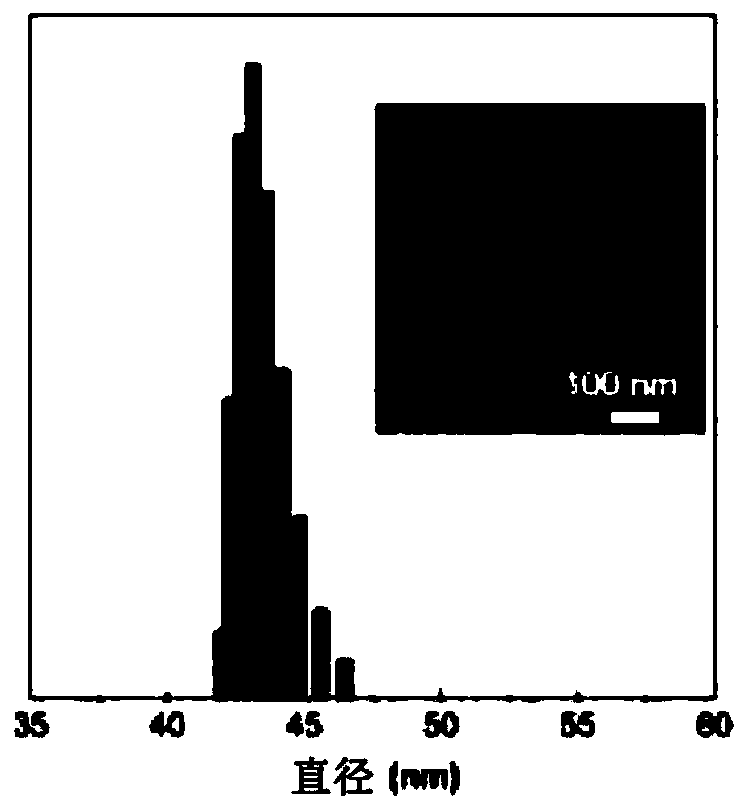
[0105] For different THF fractions (f T ) The study of the AIE characteristics of TTVP in the water / THF mixture shows that TTVP is a typical AIE active molecule ( Figure 1A ). The unimolecular state of TTVP in aqueous solution hardly emits light, and when the fraction of THF increases, the photoluminescence (PL) intensity gradually increases due to the formation of aggregates ( Figure 1C ). In t...
Embodiment 3
[0112] cell imaging
[0113] As a water-soluble NIR-luminescent AIE emitter, TTVP maintains an "off" state in an aqueous environment. Thus, TTVP can be used as a "light-up" probe for biological imaging with minimal background interference from both free dye and biological substrate autofluorescence. In preliminary bioimaging experiments, HeLa cells were used as cell models and incubated in 500 nM TTVP for 10 minutes for cell imaging studies. It was observed that regardless of whether the cells were washed after staining, the plasma membrane of the cells could be clearly visualized with excellent image contrast with the cell background ( Figures 6A to 6B ). The effect on the incubation period was then studied by using a no-wash program with different staining times. The results showed that the staining time was shortened from 10 minutes to 30 seconds without significant changes in fluorescence imaging quality in terms of both fluorescence intensity and specificity ( Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com