Thermally activated delayed fluorescence material and its preparation method, organic electroluminescent device
A thermally activated delay and fluorescent material technology, applied in the direction of luminescent materials, electrical solid devices, chemical instruments and methods, etc., can solve the problems affecting the stability of OLED devices, the decline of OLED device efficiency, and the fast efficiency decay speed, etc., to achieve enhanced absorption The effect of electronic ability, reduction of interaction, and improvement of light extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
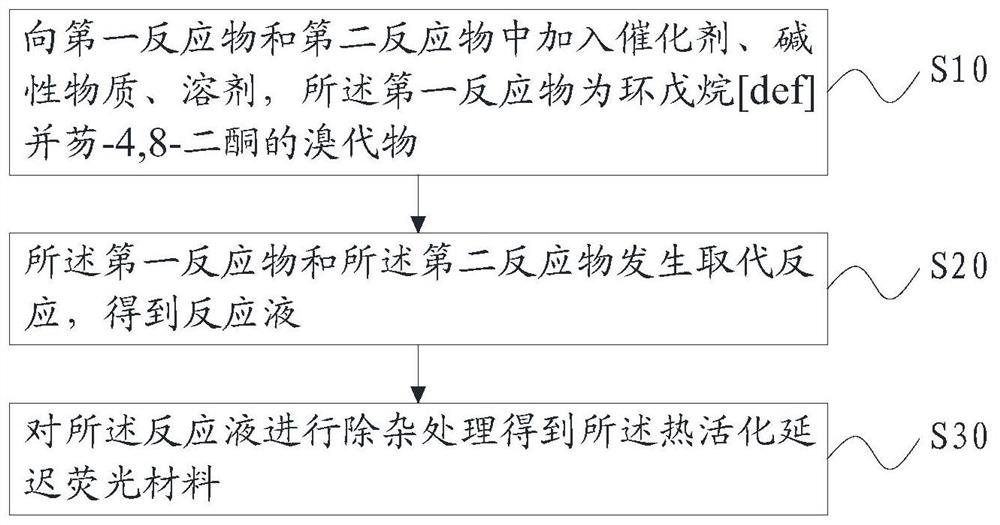
[0040] Such as figure 1 As shown, based on the above purpose, the embodiment of the present application also provides a preparation method of the above-mentioned thermally activated delayed fluorescent material, which includes the following steps:
[0041] S10, adding a catalyst, a basic substance, and a solvent to the first reactant and the second reactant, the first reactant being a bromide of cyclopentane[def]fluorene-4,8-dione;
[0042] S20, a substitution reaction occurs between the first reactant and the second reactant to obtain a reaction solution;
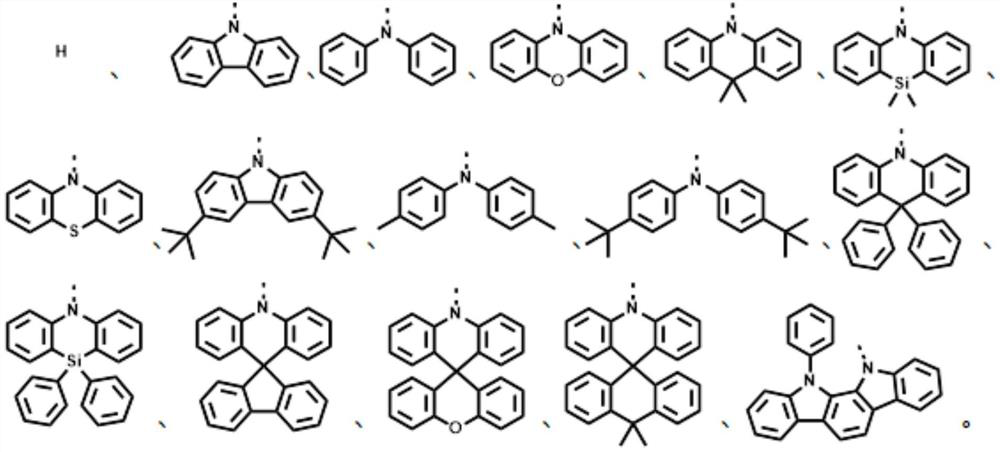
[0043] S30, performing impurity removal treatment on the reaction solution to obtain the general structural formula: The thermally activated delayed fluorescence material, R 1 -R 6 is the electron donor unit.
[0044] The second reactant can be R 1 hydride, R 2 hydride, R 3 hydride, R 4 hydride, R 5 The hydride, and R 6 Any one or more combinations of the hydrides.
[0045] The catalyst may be a palladium catal...
Embodiment 1
[0054] The first reactant in this example is 2,6-dibromocyclopentane[def]fluoren-4,8-dione, and the second reactant is 9,10-dihydro-9,9-dimethyl Acridine, the catalyst is three (dibenzylideneacetone) dipalladium, the catalyst ligand is two (2-diphenylphosphophenyl) ethers, and the molar ratio of the first reactant and the second reactant is 1:2.1 . The first reactant and the second reactant have a substitution reaction to synthesize the target compound 1, and the structural formula of the target compound 1 is: The synthetic route of target compound 1 is as follows:
[0055]
[0056] Specifically, 2,6-dibromocyclopentane[def]fluorene-4,8-dione (3.64g, 10mmol), 9,10-dihydro-9,9-dimethyl Acridine (4.40g, 21mmol), tris(dibenzylideneacetone)dipalladium (92mg, 0.1mmol), bis(2-diphenylphosphophenyl)ether (108mg, 0.2mmol) and sodium tert-butoxide (2.0g, 21mmol), pumped three times, injected 80mL of anhydrous toluene under argon atmosphere, and then reacted at 80°C for 24 hours....
Embodiment 2
[0068] The synthesis method of the target compound 2 provided in this example is the same as in Example 1, except that the first reactant is 1,7-dibromocyclopentane[def]fluorene-4,8-dione, and the target compound The structural formula of 2 is The synthetic route of target compound 2 is as follows:
[0069]
[0070]Specifically, 1,7-dibromocyclopentane[def]fluorene-4,8-dione (3.64g, 10mmol), 9,10-dihydro-9,9-dimethyl Acridine (4.40g, 21mmol), tris(dibenzylideneacetone)dipalladium (92mg, 0.1mmol), bis(2-diphenylphosphophenyl)ether (108mg, 0.2mmol) and sodium tert-butoxide (2.0g, 21mmol), pumped three times, injected 80mL of anhydrous toluene under argon atmosphere, and then reacted at 80°C for 24 hours. After the reaction was cooled to room temperature, the reaction solution was poured into 200 mL of saturated brine, and a red solid was obtained by suction filtration, which was separated and purified by column chromatography (eluent: dichloromethane:n-hexane, v:v, 1:1), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com