Bipolar electrode plate for preparing metal aluminum by aluminum chloride electrolysis and a method of using same
A bipolar electrode and metal aluminum technology, applied in the field of electrolytic aluminum, can solve the problems of reducing current efficiency and increasing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
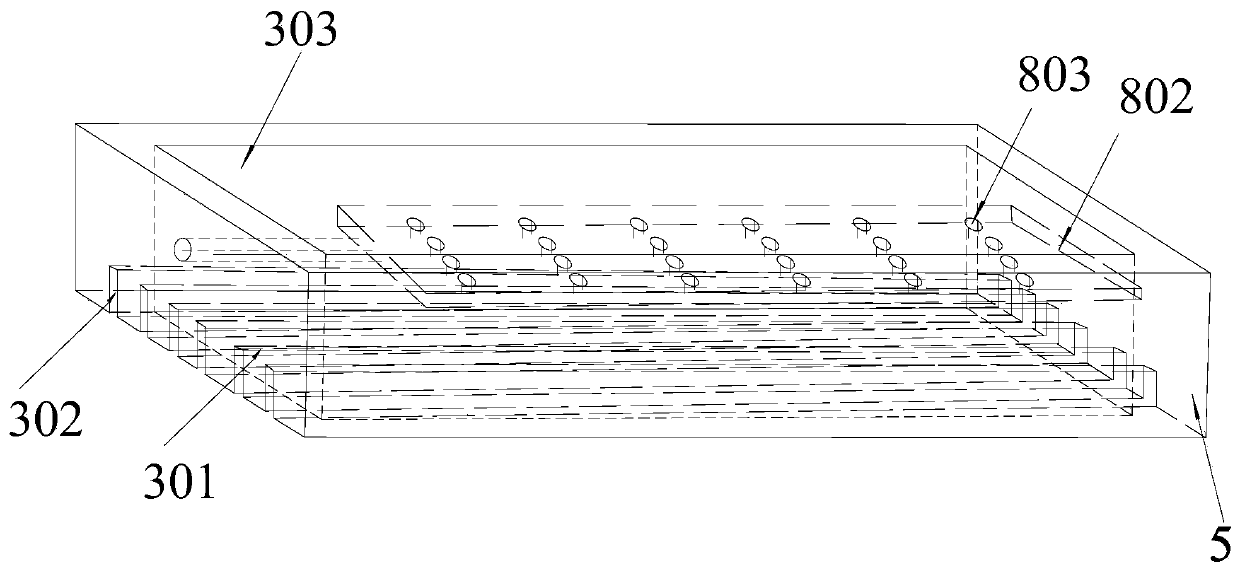
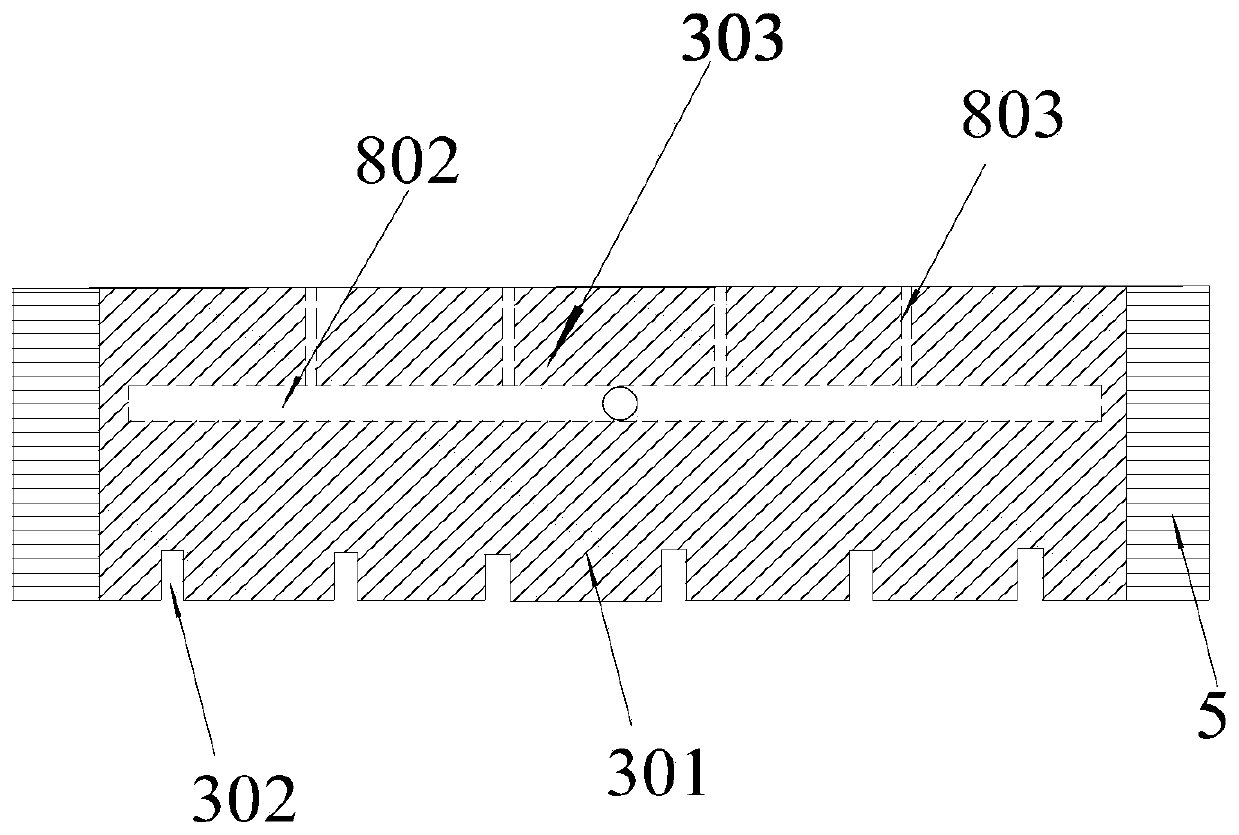
[0047] A bipolar electrode plate for the electrolysis of aluminum chloride to prepare metal aluminum, the schematic diagram of its three-dimensional structure is shown in figure 1 , see its left view figure 2 , specifically including a bipolar electrode plate base block 3 and an insulating block 5, the bipolar electrode plate base block 3 is divided into a cathode end 303 and an anode end 301, in the bipolar electrode plate base block 3, except for the cathode end 303 and the anode end 301 The end face of the anode end 301, and the remaining surroundings are provided with connecting insulating blocks, and the width of the insulating blocks needs to satisfy the following relationship:
[0048] (ρ C ·h+2.15 / D·S)L ·(b+h)
[0049] In the above formula, ρ C is the resistivity of the base of the bipolar electrode plate, in Ω cm; h is the thickness of the base of the bipolar electrode plate, in cm; ρ L is the resistivity of the electrolyte, the unit is Ω cm; D is the current den...
Embodiment 2
[0063] A bipolar electrode plate for the electrolysis of aluminum chloride to prepare metal aluminum, the schematic diagram of its three-dimensional structure is shown in Figure 4 , specifically including a bipolar electrode plate base block 3 and an insulating block 5, the bipolar electrode plate base block 3 is divided into a cathode end 303 and an anode end 301, in the bipolar electrode plate base block 3, except for the cathode end 303 and the anode end 301 On the end face of the anode end 301, an insulating block is provided on the outer periphery of the bipolar electrode plate and the wall of the central hole, and the width of the insulating block needs to satisfy the following relationship:
[0064] [ρ C ·h+2.15 / D·S]L ·(b+h)
[0065] In the above formula, ρ C is the resistivity of the base of the bipolar electrode plate, in Ω cm; h is the thickness of the base of the bipolar electrode plate, in cm; ρ L is the resistivity of the electrolyte, the unit is Ω cm; d is th...
Embodiment 3
[0085] A bipolar electrode plate for preparing metal aluminum by electrolysis of aluminum chloride is the same as in Example 1.
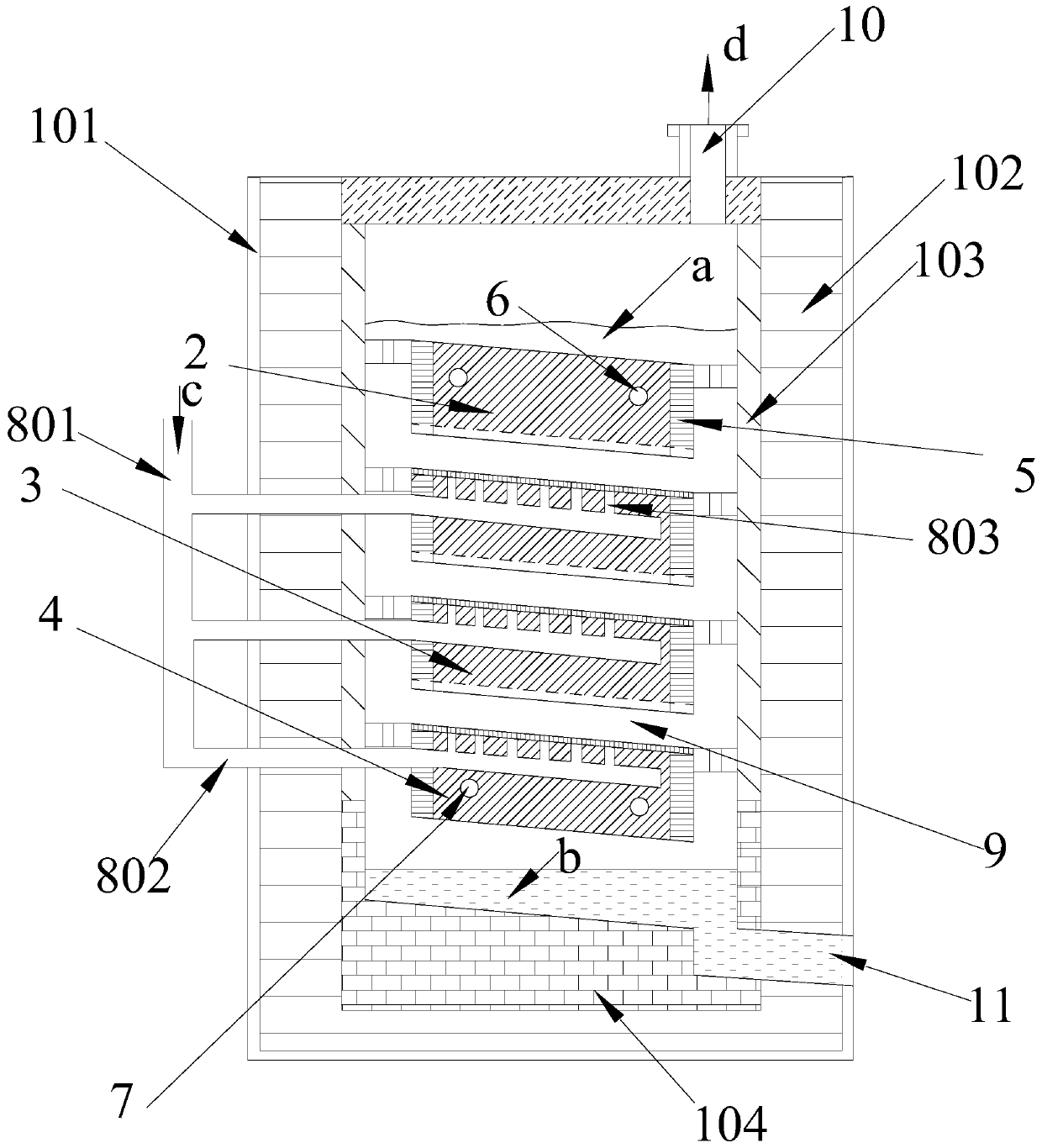
[0086] An electrolytic cell based on aluminum chloride electrolysis in this embodiment to prepare a bipolar electrode plate of metal aluminum, which is a square electrolytic cell structure, such as Figure 8 As shown, the electrolytic cell includes a shell, which is made of double-layer material, the outer layer is a stainless steel layer 101, and the stainless steel layer 101 is lined with refractory bricks. The system has a certain corrosion resistance. The lower part of the electrolytic cell is the aluminum storage area, which is used to store the liquid aluminum liquid flowing down from the cathode. In the aluminum storage area, the bottom and side walls of the shell are made of carbon material layer 104, and the carbon material layer is made of graphite.
[0087] The housing on the top of the electrolyzer is provided with an outlet for gettin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com