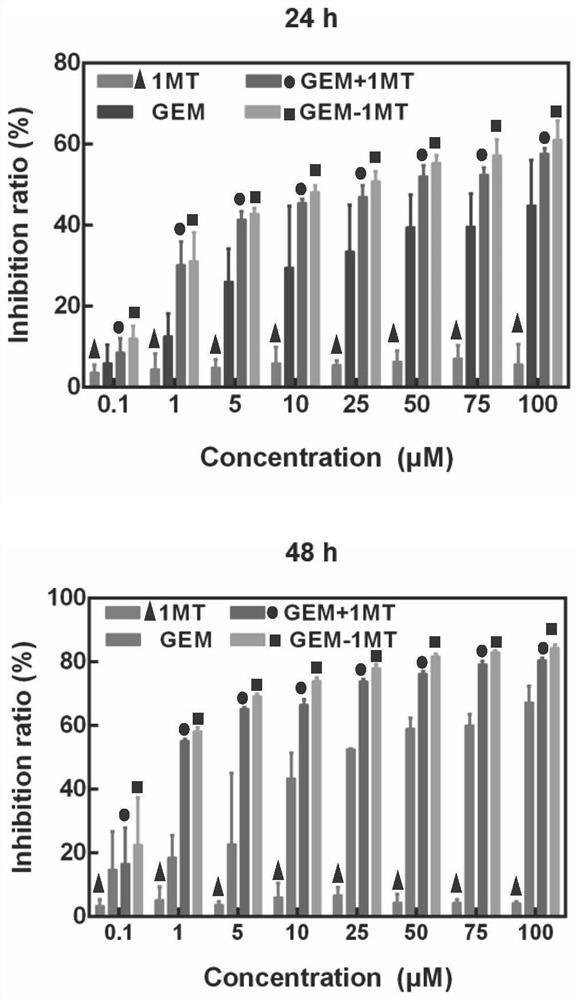
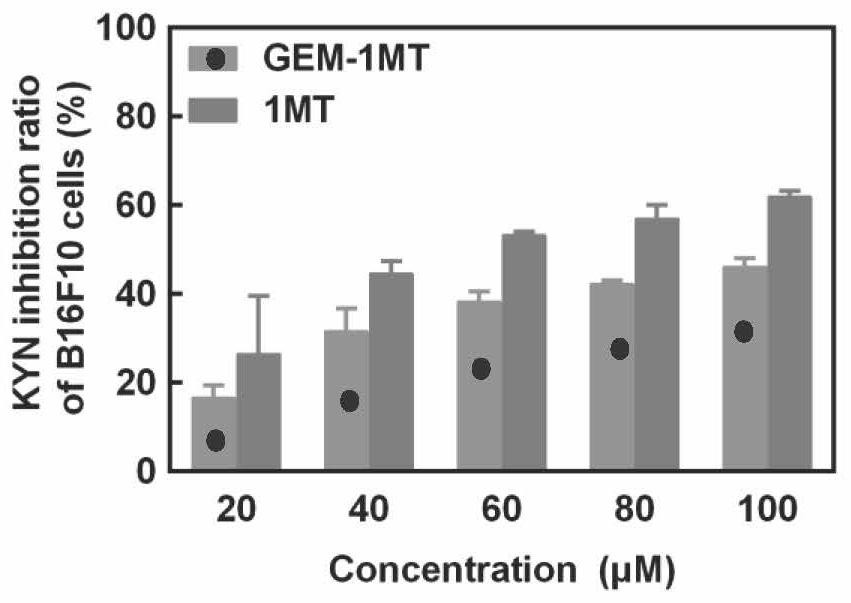
gem-1mt amphiphilic small molecule compound and its preparation, preparation method and application
A small molecule compound and compound technology, applied in the field of medicine, can solve the problems of difficult drug delivery, GEM drug resistance, strong hydrophobicity, etc., and achieve the goal of reshaping the tumor immune microenvironment, high cell internalization efficiency, and high cell inhibition rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Synthesis of GEM-1MT
[0056] A certain amount of Boc-1MT was accurately weighed with an analytical balance, dissolved in anhydrous dichloromethane, and placed in a round bottom flask. Weigh a certain amount of NHS and EDC, dissolve it in a mixed solvent of anhydrous N,N-dimethylformamide (DMF) and ethanol, add it to the stirring solution of Boc-1MT above, under nitrogen protection, at room temperature React for 12 hours. Among them, the ratio of Boc-1MT: NHS: EDC is 1:2:2. After 12 hours, accurately weigh a certain amount of 4-N-3'-O-bis(Boc)GEM and DMAP with an analytical balance, dissolve in Anhydrous dichloromethane was added dropwise to the above-mentioned mixed solution after the reaction, and the reaction was continued at room temperature for 48 hours. After the reaction was completed, anhydrous DMF, dichloromethane and ethanol were removed by rotary evaporation under reduced pressure, and dried in vacuo overnight to obtain a crude product. The cru...
Embodiment 2
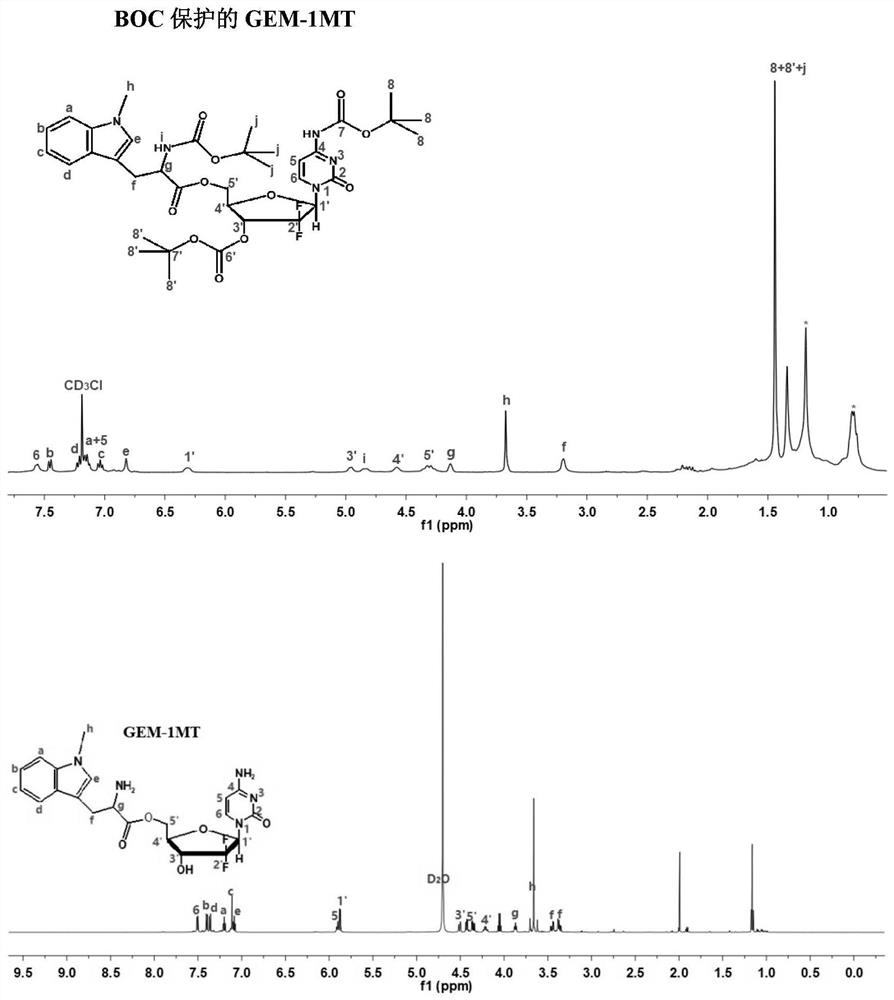
[0059] Example 2 H NMR spectrum ( 1 H-NMR) identification of Boc-protected GEM-1MT and the chemical structure of GEM-1MT
[0060] Take by weighing about 5 mg each of GEM-1MT and GEM-1MT protected by Boc prepared by the method of Example 1, and use deuterated chloroform (DMSO-d6) and deuterated water (D 2 O) dissolved and placed in a nuclear magnetic tube, using a 400MHz proton nuclear magnetic resonance spectrometer to measure its proton nuclear magnetic resonance spectrum, and record the chemical shift value (ppm) of the compound. The result is as figure 1 As shown, the NMR results can confirm that the peaks of the raw material molecules GEM and 1MT appear in the newly synthesized molecules, confirming the successful synthesis of Boc-protected GEM-1MT and GEM-1MT.
Embodiment 3
[0061] Example 3 Preparation of GEM-1MT NPs
[0062] Accurately weigh about 10 mg of GEM-1MT prepared by the method in Example 1, and dissolve it in 200 μL of methanol. Then, under ultrasonic conditions, it was added dropwise to 1 mL of water. The mixture was continued to be sonicated for half an hour, and nanoaggregates formed spontaneously. Finally, methanol was completely evaporated at 30°C using a rotary evaporator under vacuum to obtain GEM-1MT NPs with a uniform particle size of about 95±5nm, suitable for intravenous injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com