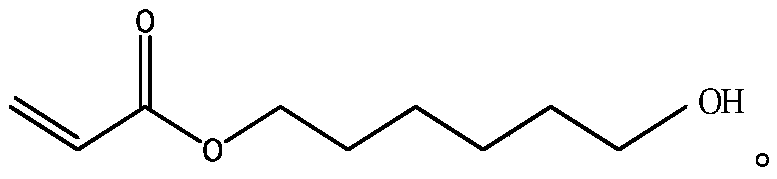
Preparation and separation method of acrylic acid-6-hydroxyhexyl ester
A separation method and technology for acrylic acid, applied in the chemical industry, can solve the problems of increased loss of hydroxyhexyl acrylate, low yield of hydroxyhexyl acrylate, inability to realize separation, etc., and achieve the effects of improving separation effect, good extraction effect and effective separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation and separation method of the 6-hydroxyhexyl acrylate of the present embodiment comprises the following steps:
[0043] (1) Put 106.2kg of 1,6-hexanediol, 36kg of acrylic acid, 0.36kg of copper N,N-dibutyldithiocarbamate and 0.72kg of p-toluenesulfonic acid into a 250L reactor, stir and heat up to 105°C for reaction 1.5h, the reaction solution was obtained. Among them, the reaction solution mainly includes the following main components: 47.6wt% of 1,6-hexanediol, 42.2wt% of 6-hydroxyhexyl acrylate, 6.3wt% of 1,6-hexanediol diacrylate, The selectivity of 6-hydroxyhexyl acrylate was 82.8%, and the conversion rate was 78.1%.
[0044] (2) Add water to the reaction solution obtained in step (1) to form water phase A, the mass ratio of water to the reaction solution is 1: 2.75; use cyclohexane as the extraction agent, and remove the reaction by 9 stages of continuous countercurrent centrifugal extraction 1,6-hexanediol diacrylate in the liquid; during the ext...
Embodiment 2
[0049] The preparation and separation method of the 6-hydroxyhexyl acrylate of the present embodiment comprises the following steps:
[0050] (1) Put 129.8kg of 1,6-hexanediol, 36kg of acrylic acid, 0.54kg of copper N,N-dibutyldithiocarbamate, and 1.26kg of phosphoric acid into a 250L reactor, stir and heat up to 110°C for 0.5h, get the reaction solution. Among them, the reaction solution mainly includes the following main components: 48.6 wt% of 1,6-hexanediol, 41.1 wt% of 6-hydroxyhexyl acrylate, and 5.2 wt% of 1,6-hexanediol diacrylate , the selectivity of acrylate-6-hydroxyhexyl ester was 84.2%, and the conversion rate was 79.3%.
[0051] (2) Add water to the reaction solution obtained in step (1) to form water phase A, the mass ratio of water to the reaction solution is 1: 3.25; use cyclohexane as the extraction agent, and remove the reaction solution through 11 stages of continuous countercurrent centrifugal extraction 1,6-hexanediol diacrylate; during the extraction p...
Embodiment 3
[0056] The preparation and separation method of the 6-hydroxyhexyl acrylate of the present embodiment comprises the following steps:
[0057] (1) Put 115kg of 1,6-hexanediol, 36kg of acrylic acid, 0.42kg of copper N,N-dibutyldithiocarbamate, and 1.1kg of methanesulfonic acid into a 250L reactor, stir and heat up to 108°C for 1 hour , to get the reaction solution. Among them, the reaction solution mainly includes the following main components: 47.9 wt% of 1,6-hexanediol, 41.6 wt% of 6-hydroxyhexyl acrylate, and 5.8 wt% of 1,6-hexanediol diacrylate , the selectivity of acrylate-6-hydroxyhexyl ester was 83.2%, and the conversion rate was 78.7%.
[0058] (2) Add water to the reaction solution obtained in step (1) to form water phase A, the mass ratio of water to the reaction solution is 1:3; use cyclohexane as the extraction agent, and remove the reaction by 10 stages of continuous countercurrent centrifugal extraction 1,6-hexanediol diacrylate in the liquid; during the extracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com