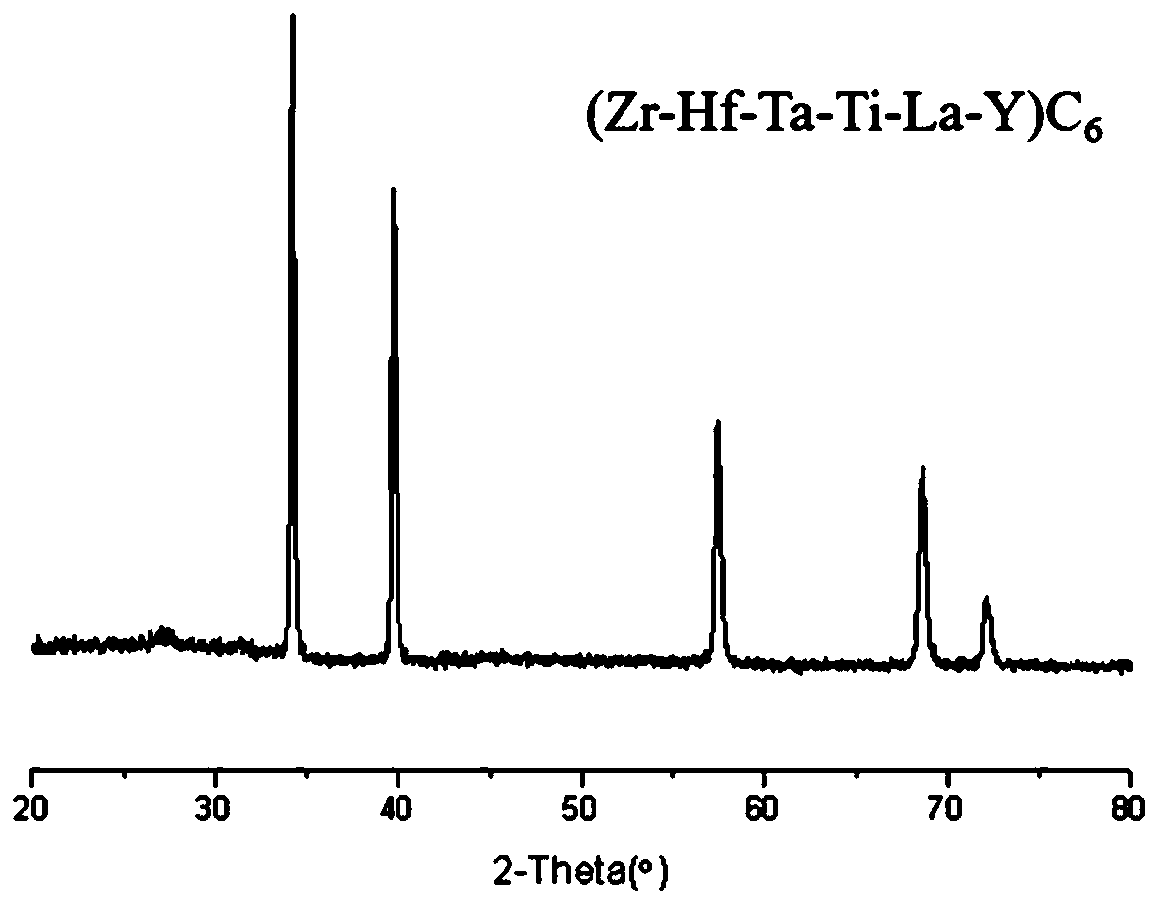
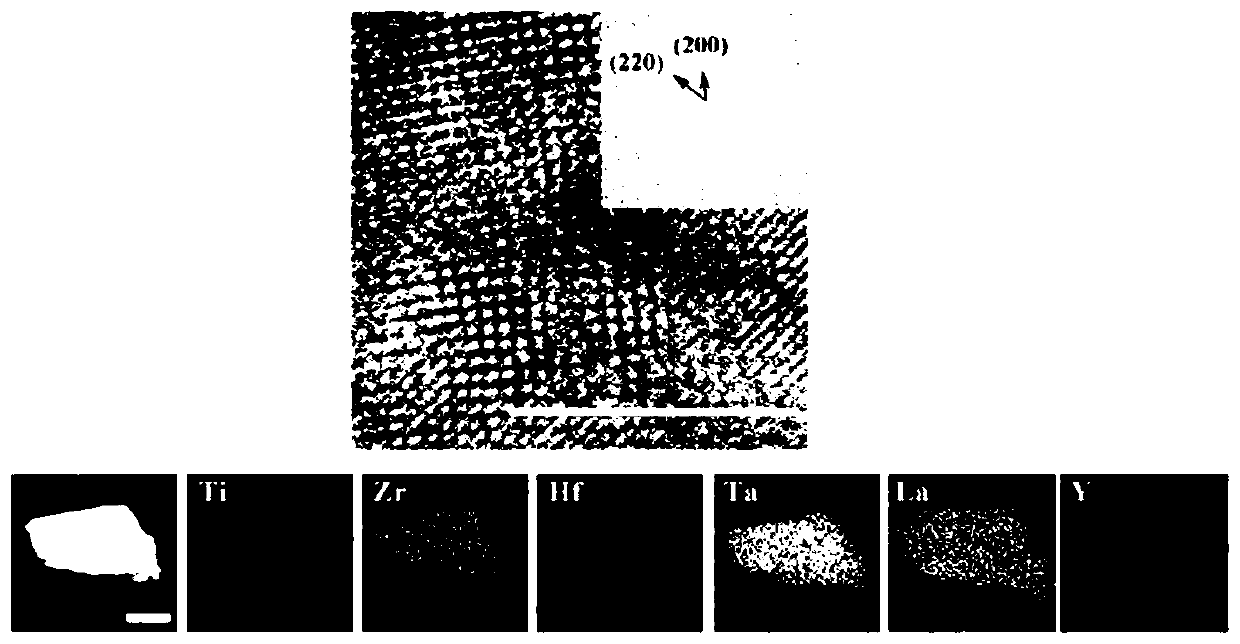
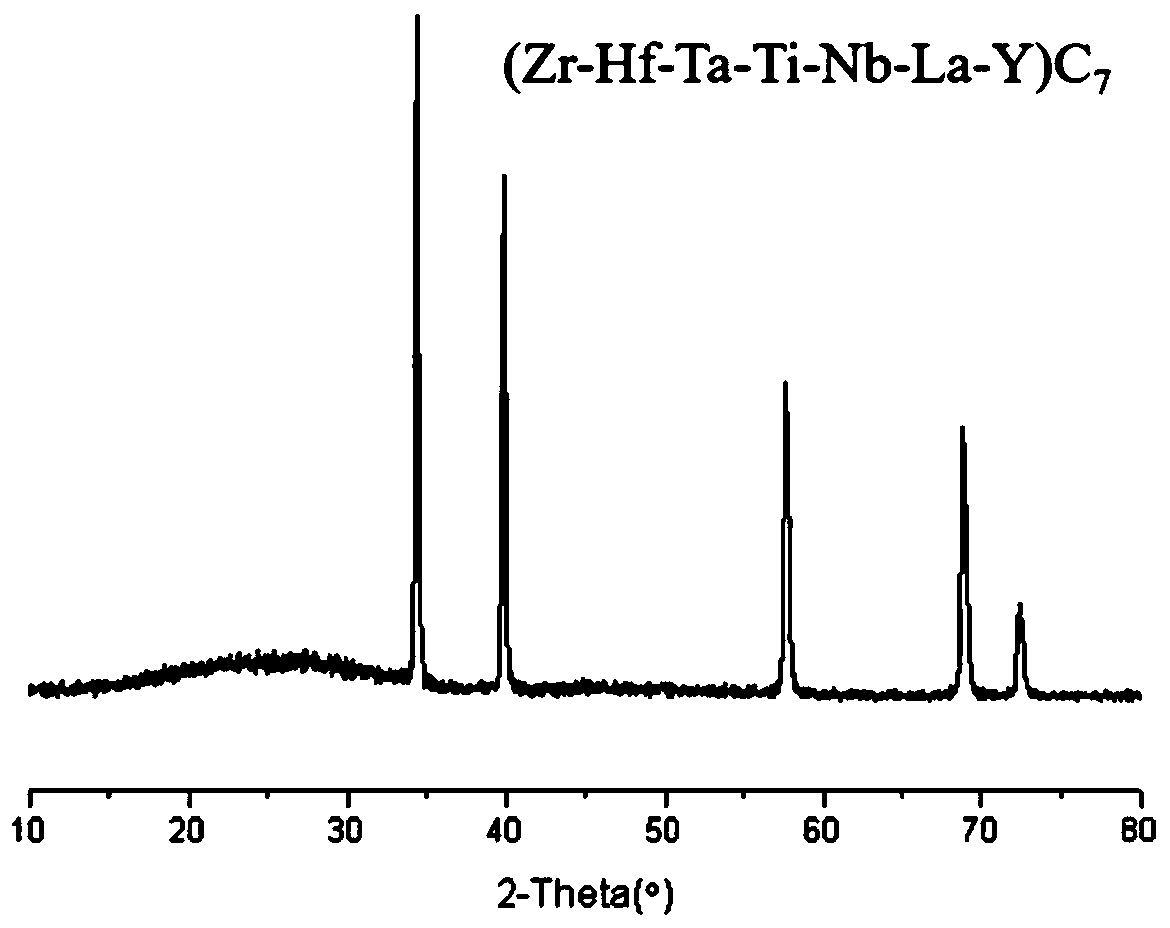
Carbide high-entropy ceramic precursor containing rare earth, high-entropy ceramic and preparation method
A ceramic precursor, rare earth carbide technology, applied in the field of high-entropy materials to achieve good storage performance and small viscosity changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In this example, the following methods are used to prepare precursors and high-entropy ceramics:
[0052] (1) Obtain metal alkoxide: select metal alkoxide Zr(OPr) 4 、Hf(OPr) 4 、Ta(OPr) 5 and Ti(OPr) 4 , where Hf(OPr) 4 、Ta(OPr) 5 is the metal salt HfCl 4 , TaCl 5 Disperse in n-heptane and ethylene glycol dimethyl ether respectively. At -10°C, add monohydric alcohol n-propanol dropwise, and then drop triethylamine respectively. After the dropwise addition, heat and reflux for 1 hour, and filter separately to obtain metal alkoxides Solution, final vacuum distillation obtains metal alkoxide; Wherein, the ratio of metal salt, monohydric alcohol and triethylamine is respectively 1:4:4, 1:5:6;
[0053] (2) Preparation of metal alkoxide complexes: under the condition of 40°C, the metal alkoxide Zr(OPr) 4 、Hf(OPr) 4 、Ta(OPr) 5 and Ti(OPr) 4Add acetylacetone dropwise, and continue to stir for 0.1h after dropping; the metal alkoxide Zr(OPr) 4 、Hf(OPr) 4 、Ta(OPr) 4 、...
Embodiment 2
[0058] In this example, the following methods are used to prepare precursors and high-entropy ceramics:
[0059] (1) Obtain metal alkoxide: select metal alkoxide Zr(OPr) 4 、Hf(OPr) 4 、Ti(Oi-Pr) 4 , Ta(OCH 2 CH 2 OCH 2 CH 3 ) 5 and Nb(OCH 2 CH 2 OCH 3 ) 5 , where Hf(OPr) 4 , Ta(OCH 2 CH 2 OCH 2 CH 3 ) 5 and Nb(OCH 2 CH 2 OCH 3 ) 5 is the metal salt HfCl 4 , TaCl 5 and NbCl 5 Disperse in n-heptane, n-hexane and ethylene glycol dimethyl ether respectively, at -10°C, drop monohydric alcohol n-propanol, ethylene glycol ethyl ether and ethylene glycol methyl ether, and then drop triethylamine respectively, After the dropwise addition, heat to reflux for 1 hour, filter to obtain the metal alkoxide solution, and finally distill under reduced pressure to obtain the metal alkoxide; wherein, the ratios of metal salt, monohydric alcohol and triethylamine are 1:4:4, 1:5:6, respectively. and 1:6:6;
[0060] (2) Preparation of metal alkoxide complexes: under the cond...
Embodiment 3
[0065] In this example, the following methods are used to prepare precursors and high-entropy ceramics:
[0066] (1) Obtain metal alkoxide: metal alkoxide Zr(OPr) 4 、Hf(OPr) 4 、Ti(Oi-Pr) 4 、Ta(OPr) 5 , Mo(OCH 2 CH 2 OCH 2 CH 3 ) 5 and W(OCH 2 CH 2 OCH 3 ) 6 , where Hf(OPr) 4 、Ta(OPr) 5 , Mo(OCH 2 CH 2 OCH 2 CH 3 ) 5 and W(OCH 2 CH 2 OCH 3 ) 6 is the metal salt HfCl 4 , TaCl 5 、MoCl 5 and WCl 6 Separately disperse in toluene, at -5°C, add monohydric alcohol n-propanol, n-propanol, ethylene glycol ethyl ether, ethylene glycol methyl ether dropwise, and then add triethylamine dropwise, heat to reflux for 1 hour after the dropwise addition, Filtrate respectively to obtain metal alkoxide solution; Wherein, the ratio of metal salt, monohydric alcohol and triethylamine is respectively 1:4:4, 1:5:6, 1:6:5, 1:8:7;
[0067] (2) Preparation of metal alkoxide complexes: under the condition of 80°C, the metal alkoxide Zr(OPr) 4 、Hf(OPr) 4 、Ti(Oi-Pr) 4 、Ta(OPr)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com