Preparation method and application of humanized LAG-3 (lymphocyte activation gene 3) modification animal model
A LAG-3, genetic modification technology, applied in the application field of biomedicine, can solve the problems of poor repeatability of test results, time-consuming, capital and scientific research effort, and inability to detect and evaluate drug efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Embodiment 1 LAG-3 gene humanized mouse
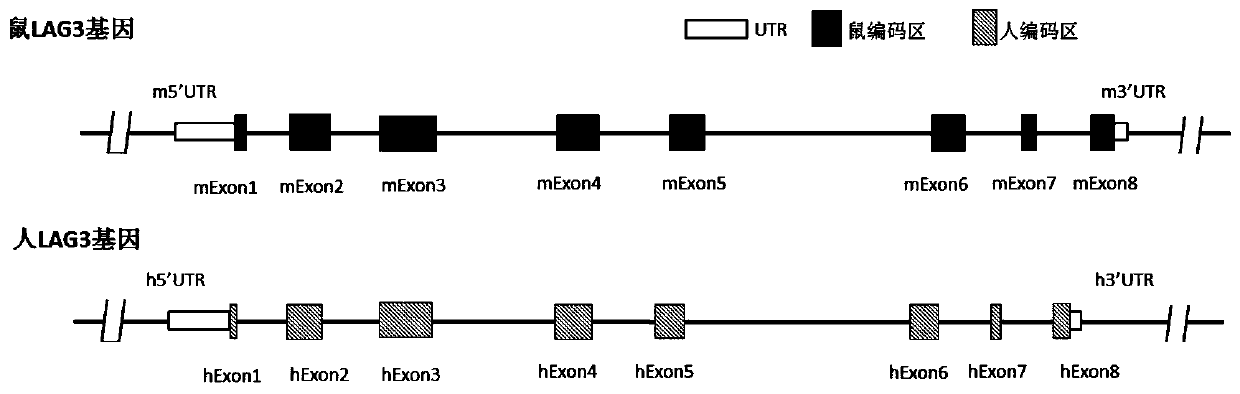
[0190] Mouse LAG-3 gene (NCBI Gene ID: 16768, Primary source: MGI: 106588, UniProtID: Q61790, located at positions 124904359 to 124912434 of chromosome 6 NC_000072.6, based on transcript NM_008479.2 (SEQ ID NO: 1 ) and its encoded protein NP_032505.1 (SEQ ID NO: 2)) and human LAG-3 gene (NCBIGene ID: 3902, Primary source: HGNC: 6476, UniProt ID: P18627, located at No. 6772483 of chromosome 12 NC_000012.12 To position 6778455, based on the comparison of the transcript NM_002286.5 (SEQ ID NO: 3) and its encoded protein NP_002277.4 (SEQ ID NO: 4)) as shown in figure 1 shown.
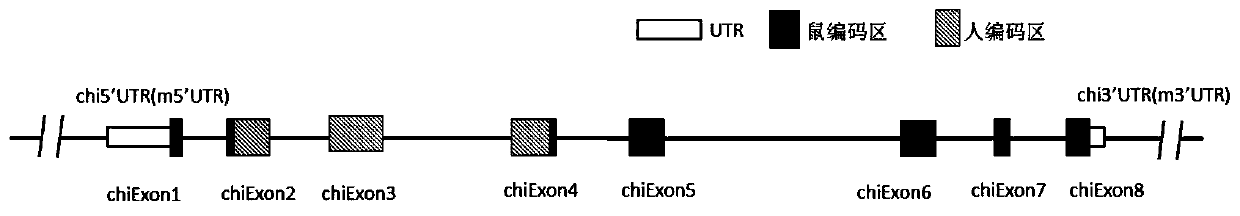
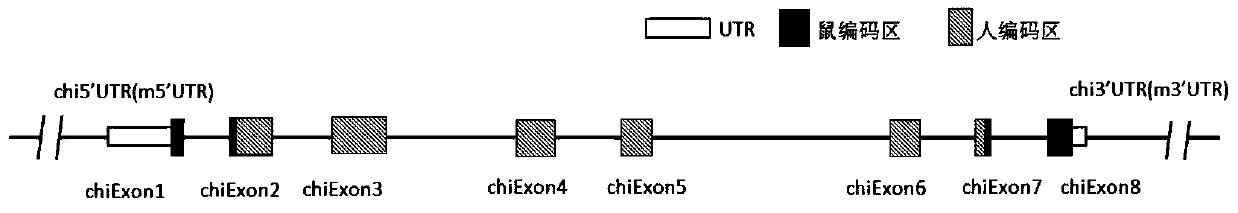
[0191]In order to achieve the purpose of the present invention, the gene sequence encoding human LAG-3 protein can be introduced into the extracellular region of the endogenous mouse LAG-3 locus, so that the mouse expresses human or humanized LAG-3 protein. Modify mouse cells with gene editing technology, replace specific mouse LAG-3 gene sequences with cer...
Embodiment 2
[0277] Example 2 In vivo efficacy verification of LAG-3 gene humanized mouse model
[0278] The tumor model constructed by using the humanized mouse prepared by the method can be used to test the drug targeting human LAG3. In one experiment, the LAG-3(s) gene humanized homozygous mice (4-6 weeks) prepared in Example 1 were subcutaneously inoculated with mouse colon cancer cells MC38 until the tumor volume was about 100mm 3 Then they were randomly divided into control group or treatment group (n=8 / group). The treatment group randomly selected an anti-human LAG-3 monoclonal antibody (AB1, obtained by immunizing mice with conventional methods, see
[0279] Janeway's Immunobiology
[0280] (9thEdition)), the dosage was 10mg / kg, and the control group was injected with normal saline. Dosing method: intraperitoneal injection, 2 times a week, 6 times in total. The tumor volume was measured twice a week, and the tumor volume of a single mouse reached 3000mm after inoculation 3 Per...
Embodiment 3
[0286] Example 3 Preparation and identification of double humanized or multiple humanized mice
[0287] Double-humanized or multiple-humanized mouse models can also be prepared by using the method or the prepared LAG-3 mice. For example, in the aforementioned Example 1, the fertilized egg cells or embryonic stem cells used in the microinjection and embryo transfer process can be selected from mice containing other genetic modifications, or can also be selected from fertilized LAG-3 humanized mice Gene editing of egg cells can further generate a double-gene or polygene-modified mouse model of LAG-3 humanization and other genetic modifications. The homozygous or heterozygous LAG-3 mice obtained by this method can also be mated with other genetically modified homozygous or heterozygous mice, and their offspring can be screened. According to the law of Mendelian inheritance, there is a certain probability of obtaining LAG-3 Humanized and other gene-modified double-gene or polygen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com