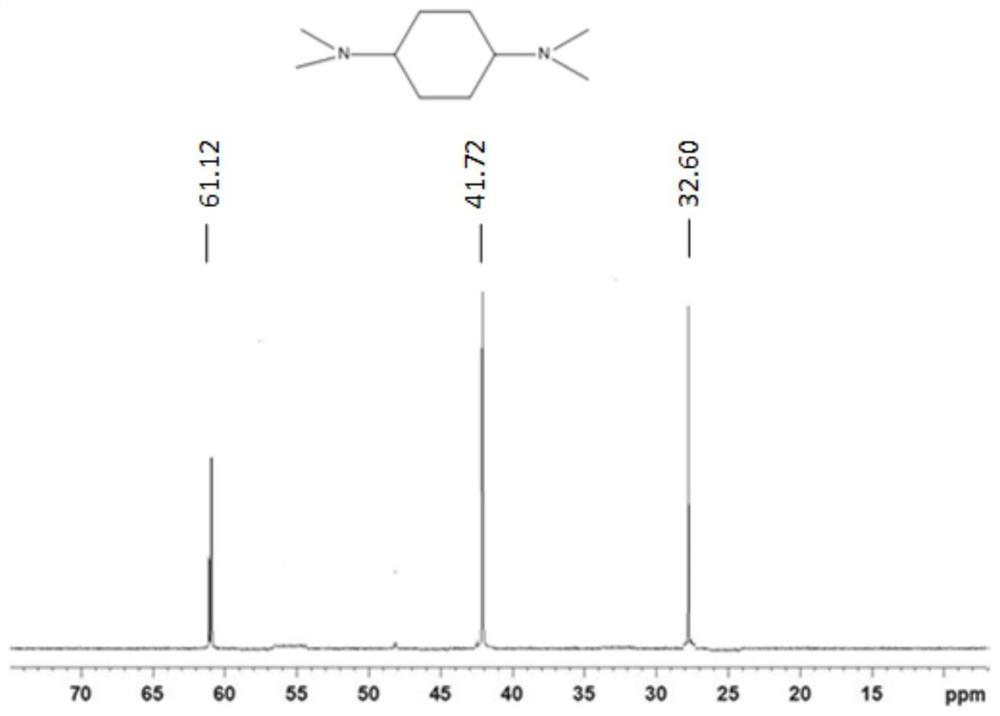
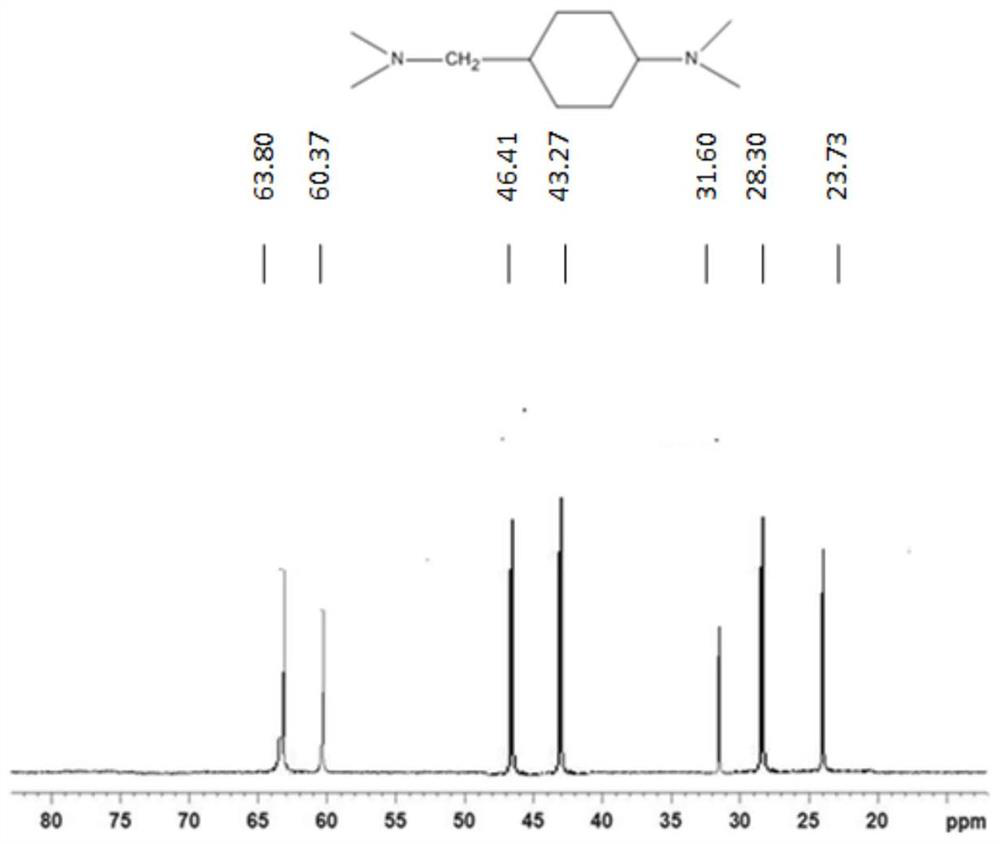
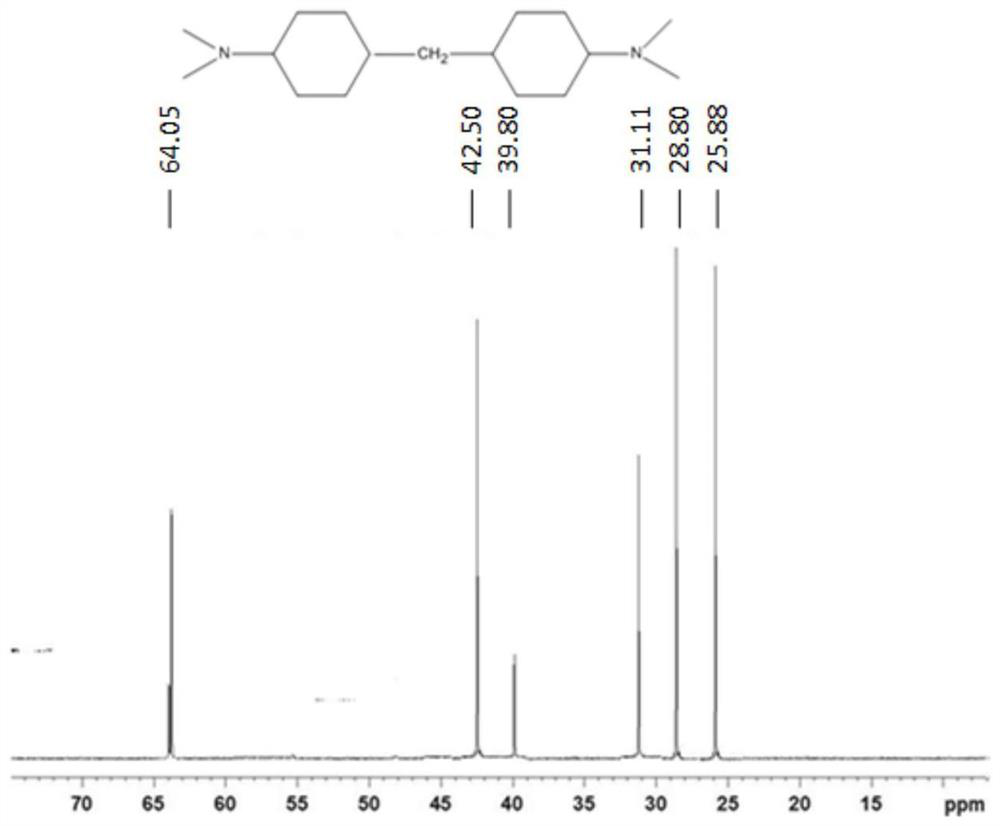
Use of n,n-dimethylcyclohexane tertiary amine derivatives as catalysts for preparing polyurethane and/or polyisocyanurate foams
A technology of dimethylcyclohexane tertiary amine and polyisocyanurate, which is applied in the field of preparation of gel-type catalyst N,N-dimethylcyclohexane tertiary amine derivatives, and can solve problems such as poor stability , to achieve the effect of stable system, good gel effect and low odor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of palladium series catalyst 1:
[0059] Dissolve 25.04g of palladium nitrate dihydrate, 0.14g of rhodium nitrate, and 2.76g of ruthenium acetate in 100ml of deionized water, heat to 60°C to form a homogeneous solution, then add 88.95g of alumina (average particle size 50μm, specific surface area 180m 2 / g, pore volume 0.30cc / g), in a water bath at 70°C for 5 hours and then gradually evaporated to dryness, then baked in an oven at 100°C for 16 hours; finally moved to a muffle furnace, in an air atmosphere at 3°C / The temperature was raised to 550°C for 8 hours, and the catalyst was obtained after natural cooling. The composition of the catalyst is as follows: Pd is 10wt%, Rh is 0.05wt%, Ru is 1wt%, and the rest is alumina. Based on the corresponding metal elements accounting for the total mass of the catalyst, the metal exists in an oxidized state and needs to be reduced during use.
Embodiment 2
[0061] Preparation of Palladium Series Catalyst 2:
[0062] Dissolve 5.01g of palladium nitrate dihydrate, 1.40g of rhodium nitrate, and 5.51g of ruthenium acetate in 100ml of deionized water, heat to 70°C to form a homogeneous solution, then add 95.5g of silicon dioxide (average particle size 60μm, specific surface area 240m 2 / g, pore volume 0.38cc / g), in a water bath at 60°C for 6 hours and then gradually evaporated to dryness, then baked in an oven at 120°C for 12 hours; finally moved to a muffle furnace, in an air atmosphere at 2°C / The temperature was raised to 600°C for 6 hours, and the catalyst was obtained after natural cooling. The composition of the catalyst is: Pd is 2wt%, Rh is 0.5wt%, Ru is 2wt%, and the rest is silicon dioxide. Based on the corresponding metal elements accounting for the total mass of the catalyst, the metal exists in an oxidized state and needs to be reduced during use.
Embodiment 3
[0064] Preparation of trans N,N,N,N-tetramethyl-1,4-cyclohexanediamine:
[0065] Add 4g of the palladium-based catalyst in Example 1 to a 1L reaction kettle, add methanol solvent as a primer, seal the reaction kettle, replace with nitrogen and hydrogen for three times, and activate the catalyst at a temperature of 240°C and a hydrogen pressure of 5MPa 6 hours. After that, the temperature was lowered, the pressure was released, and nitrogen was replaced three times, and the solvent was filtered out from the reaction kettle. Then add 100g of trans-1,4-cyclohexanediamine, replace with nitrogen and hydrogen three times in sequence, the initial hydrogen pressure is 2MPa, start stirring at 700 rpm, wait for the reaction temperature to rise to 160°C, and put the hydrogen Adjust the pressure to 3MPa and continue to flow hydrogen, start to flow 170g of 37% formaldehyde solution into the reactor at a speed of 2g / min with a convection pump, wait until the instantaneous flow rate of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com