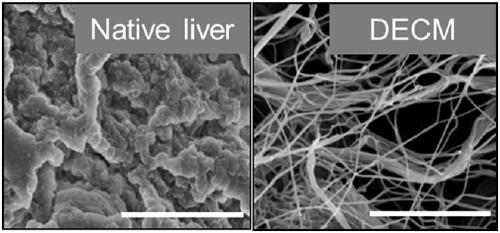
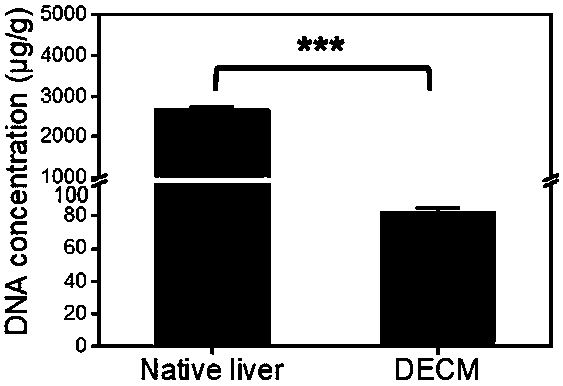
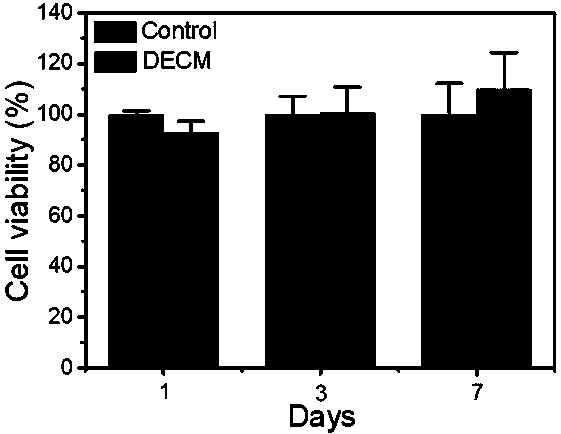
Decellularized extracellular matrix (DECM) template used for simulating liver cancer environment
A decellularization and template technology, applied in the direction of liver cells, tumors/cancer cells, cell culture supports/coatings, etc., can solve the problems of time-consuming and long-term cycle, materials are not easy to obtain, etc., achieve low price, promote proliferation and Adhesive, easy handling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] (1) Combine DPPC, DPPE, DPPA, DSPE-PEG 2000 and Fe 3 o 4 Dissolve in a mixed solution of 10 mL methanol and 5 mL chloroform, and obtain solution S1 by ultrasonication, in which the concentration of each substance is 2 mg / mL DPPC, 5 mg / mL DPPE, 5 mg / mLDPPA, 5 mg / mL DSPE- PEG 2000 and 1.33 mg / mL Fe 3 o 4 ;
[0064] (2) After ultrasonication for 1 min, the solution S1 was rotary evaporated at 55 °C for 1 h to form a film to obtain P-FeNBs;
[0065] (3) Mix 500 µL glycerol, 5 mL PBS and 3 mg F-68 to obtain hydration solution S2;
[0066] (4) Add the hydration solution S2 into the P-FeNBs, and ultrasonically dissolve the liposome membrane for 5 min to obtain FeNBs;
[0067] (5) DOX was added to the obtained FeNBs solution in an amount of 3.64 mg / mL, and after 1 h in an oil bath at 75 °C, it was shaken at 500 rpm for 2 h in a perfluoropropane atmosphere, and centrifuged at 800 rpm for 10 min, centrifuged and washed 3 times with PBS at a centrifugation speed of 20,000 rp...
Embodiment 2
[0083] (1) Combine DPPC, DPPE, DPPA, DSPE-PEG 2000 and Fe 3 o 4 Dissolve in a mixed solution of 80 mL methanol and 1 mL chloroform, and obtain solution S1 by ultrasonication, in which the concentration of each substance is 5 mg / mL DPPC, 2 mg / mL DPPE, 4 mg / mLDPPA, 0.02 mg / mL DSPE- PEG 2000 and 0.01 mg / mL Fe 3 o 4 ;
[0084] (2) After ultrasonication for 5 min, the solution S1 was rotary evaporated at 20 °C for 10 min to form a film to obtain P-FeNBs;
[0085] (3) Mix 1 µL glycerol, 10 mL PBS and 15 mg F-68 to obtain hydration solution S2;
[0086] (4) Add the hydration solution S2 into the P-FeNBs, and ultrasonically dissolve the liposome membrane for 20 min to obtain FeNBs;
[0087] (5) DOX was added to the obtained FeNBs solution in an amount of 0.005 mg / mL. After 3 h in an oil bath at 100 °C, it was shaken at 300 rpm for 5 h in a perfluoropropane atmosphere, and centrifuged at 800 rpm for 5 h. min, washed once with PBS at a centrifugation speed of 3000 rpm for 1 min to...
Embodiment 3
[0094] (1) Combine DPPC, DPPE, DPPA, DSPE-PEG 2000 and Fe 3 o 4 Dissolve in a mixed solution of 40 mL methanol and 20 mL chloroform, and obtain solution S1 by ultrasound, in which the concentration of each substance is 5 mg / mL DPPC, 4.8 mg / mL DPPE, 2 mg / mLDPPA, 0.02 mg / mL DSPE- PEG 2000 and 0.005 mg / mL Fe 3 o 4 ;
[0095] (2) After ultrasonication for 5 min, the solution S1 was rotary evaporated at 20 °C for 10 min to form a film to obtain P-FeNBs;
[0096] (3) Mix 1 µL glycerol, 10 mL PBS and 15 mg F-68 to obtain hydration solution S2;
[0097] (4) Add the hydration solution S2 into the P-FeNBs, and ultrasonically dissolve the liposome membrane for 20 min to obtain FeNBs;
[0098] (5) DOX was added to the obtained FeNBs solution in an amount of 0.005 mg / mL, and after 3 h in an oil bath at 100 °C, it was shaken at 300 rpm for 5 h in a perfluoropropane atmosphere, and centrifuged at 4000 rpm for 40 min, washed once with PBS at a centrifugation speed of 3000 rpm for 1 min ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com