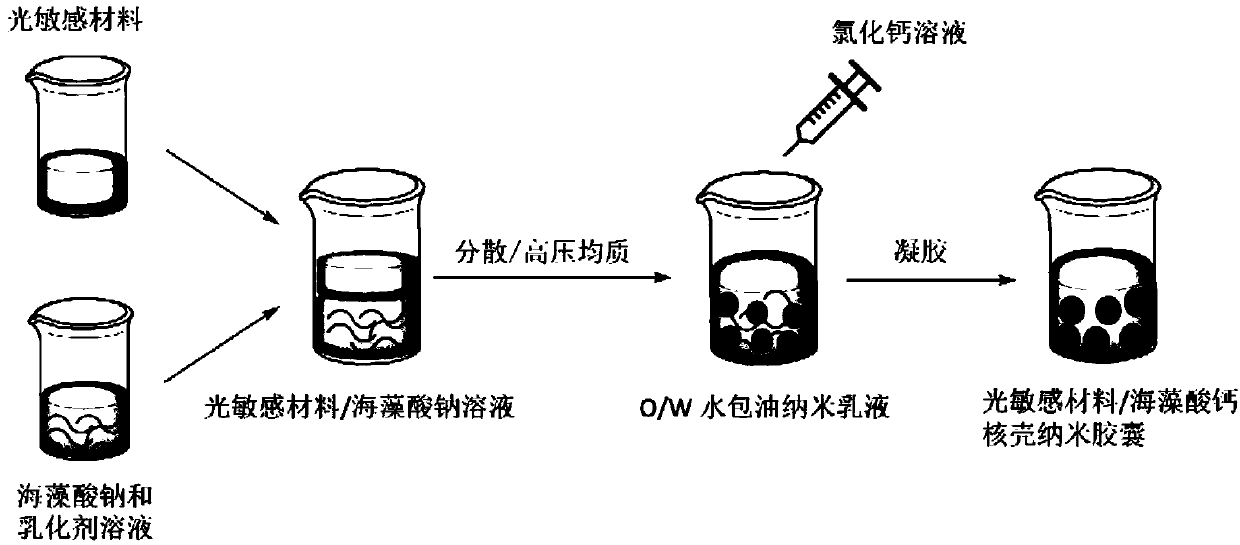
Light sensitive material/calcium alginate core-shell nanocapsule dispersoid and preparation method thereof
A technology of calcium alginate and nanocapsules, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, cosmetic preparations, etc., can solve the problems of complex preparation methods, high product costs, and expensive liposomes, and achieve the goal of preparing The effect of simple process and high wrapping rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Dissolve 0.4g SA (sodium alginate) and 0.4g PVA (emulsifier) in 40mL water to form SA aqueous solution;
[0076] Dissolve 7.5g VE in 20mL mineral oil to form VE solution (vitamin E solution);
[0077] Pour the vitamin E solution into the SA aqueous solution and disperse it with a disperser (IKA Ultra Turrax T-18, 10,000r / min, 5min) to obtain a VE emulsion;
[0078] Dissolve 0.06 g of calcium chloride in 8 mL of water, add dropwise to the VE emulsion, and stir for 15 min to obtain a vitamin E / calcium alginate capsule dispersion.
[0079] Dynamic light scattering measurements showed that the vitamin E / calcium alginate microcapsules had a particle size of 1126nm and a PDI of 0.258. Ultraviolet spectrophotometer measurement shows that the encapsulation rate of vitamin E is 95%. The optical microscope image of the obtained vitamin E / calcium alginate capsules is as figure 2 As shown, the core-shell capsules are uniform in size. Vitamin E encapsulated in calcium alginat...
Embodiment 2
[0081] Dissolve 0.4g SA (sodium alginate) and 2g Tween 20 (emulsifier) in 40mL water to form SA aqueous solution;
[0082] Dissolve 7.5g of VE in 20mL of mineral oil to form a VE solution;
[0083] Pour the vitamin E solution into the SA aqueous solution, disperse it with a disperser (IKA Ultra Turrax T-18) for 5 minutes, and the shear rate is 10,000r / min, and then use a high-pressure homogenizer (Avestin EmulsiFlex C-5) to homogenize for 10 minutes Get VE emulsion;
[0084] Dissolve 0.06 g of calcium chloride in 8 mL of water, add dropwise to the VE emulsion, and stir for 15 min to obtain a vitamin E / calcium alginate capsule dispersion.
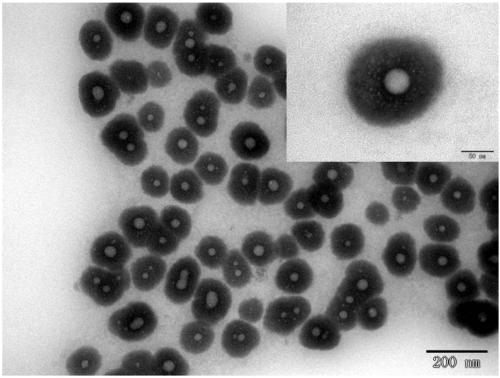
[0085] The dynamic light scattering test results are as follows Figure 5 As shown, the test results show that the vitamin E / calcium alginate nanocapsules have a particle size of 348nm and a PDI of 0.159. Ultraviolet spectrophotometer measurement shows that the encapsulation rate of vitamin E is 94%. The transmission electron microscop...
Embodiment 3
[0087] Dissolve 0.4g SA (sodium alginate) and 4g Tween 20 (emulsifier) in 40mL water to form SA aqueous solution;
[0088] Dissolve 7.5g of VE in 20mL of mineral oil to form a VE solution;
[0089] Pour the vitamin E solution into the SA aqueous solution, disperse it with a disperser (IKA Ultra Turrax T-18) for 5 minutes, and the shear rate is 10,000r / min, and then use a high-pressure homogenizer (Avestin EmulsiFlex C-5) to homogenize for 10 minutes Get VE emulsion;
[0090] Dissolve 0.06 g of calcium chloride in 8 mL of water, add dropwise to the VE emulsion, and stir for 15 min to obtain a vitamin E / calcium alginate capsule dispersion.
[0091] Dynamic light scattering tests such as Figure 5 As shown, the test results show that the vitamin E / calcium alginate nanocapsules have a particle size of 210nm and a PDI of 0.118. Ultraviolet spectrophotometer measurement shows that the encapsulation rate of vitamin E is 96%. Vitamin E encapsulated in calcium alginate nanocapsul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com