Preparation method of substituted butyrate derivatives
A technology of derivatives and butyric acid is applied in the field of preparation of substituted butyrate derivatives, which can solve the problems of low thermodynamic efficiency, large substrate restriction, and many steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]
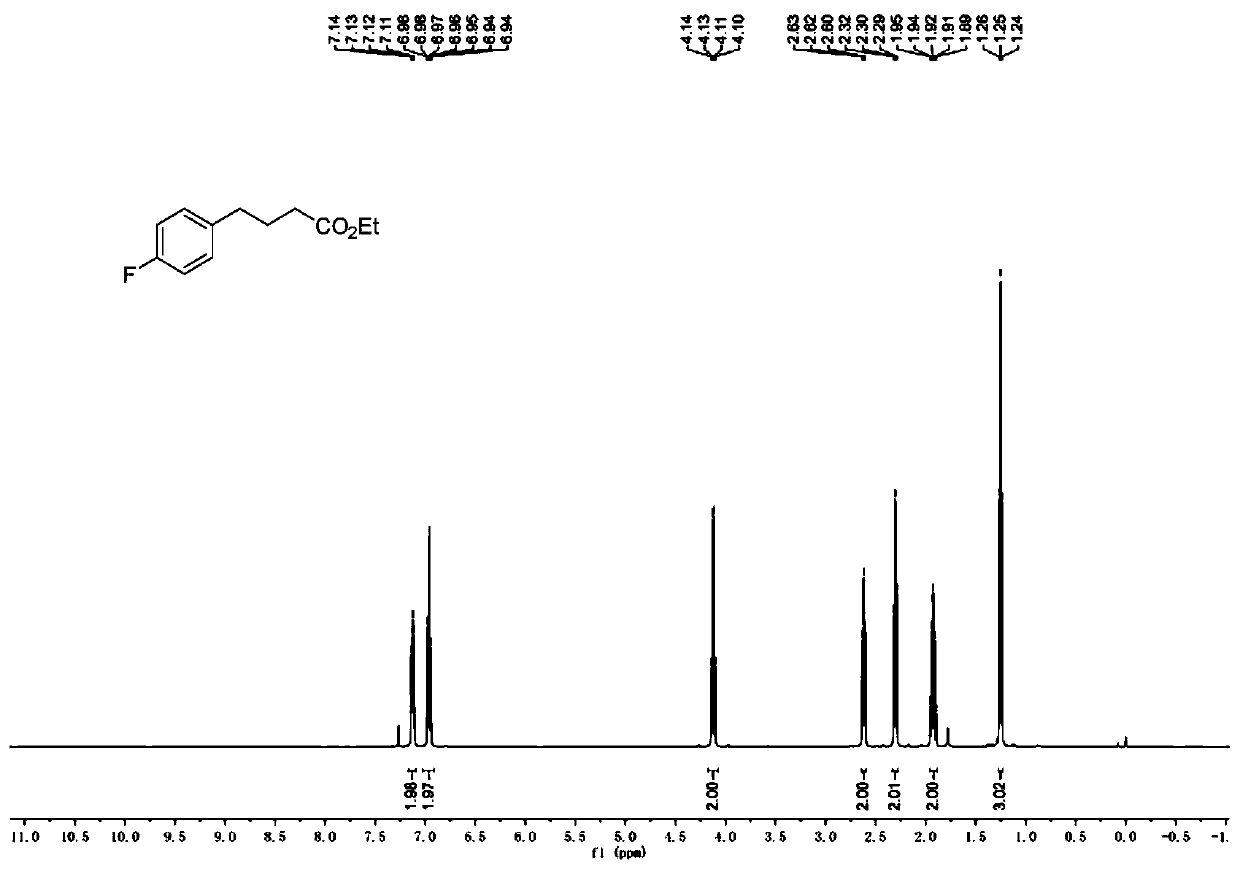
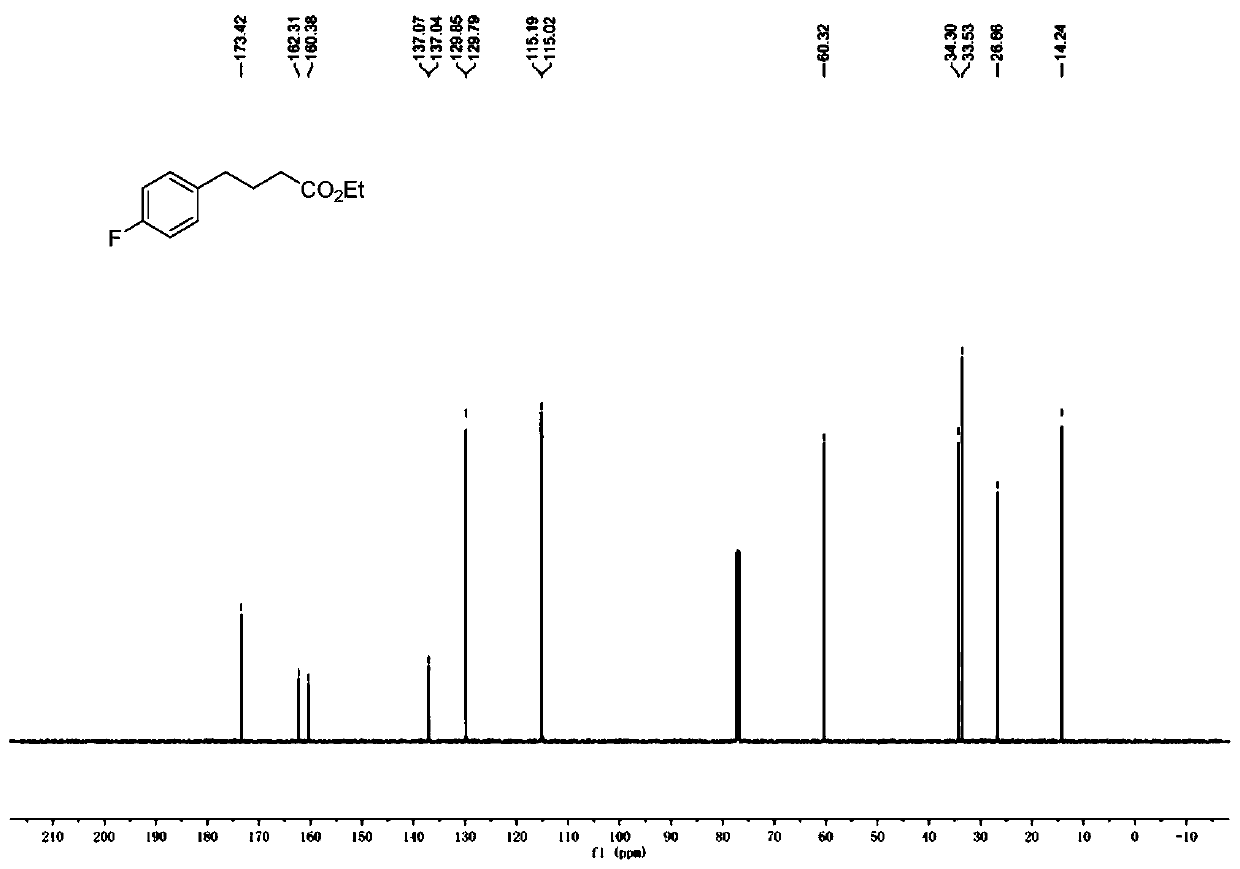
[0079] Add Hans ester Et-HE (0.8mmol), Ru (bpy) to the reaction tube equipped with a magnetic stirrer 3 Cl 2 ·6H 2 O (0.004mmol) and p-thiocresol (0.08mmol). The reaction tube was capped, and after the nitrogen had been replaced three times, 4-fluorostyrene (0.4 mmol), ethyl diazoacetate (0.8 mmol) and anhydrous dichloromethane (2.0 mL, 0.2 M) were injected into the reaction tube with a micro syringe. Then place the reaction tube at a distance of about 15 cm from a 50W blue light source for illumination and stirring. The reaction was carried out at 25° C. for 12 hours until the diazo was completely consumed (monitored by TLC). After removing the solvent by rotary evaporation under reduced pressure, the crude product was obtained. The crude product was purified by silica gel column chromatography to obtain the corresponding product (petroleum ether:ethyl acetate=30:1), the total yield: 87%.
[0080] 1 H NMR (500MHz, CDCl 3 )δ7.16–7.10(m,2H),6.99–6.93(m,2H),4....
Embodiment 2
[0082]
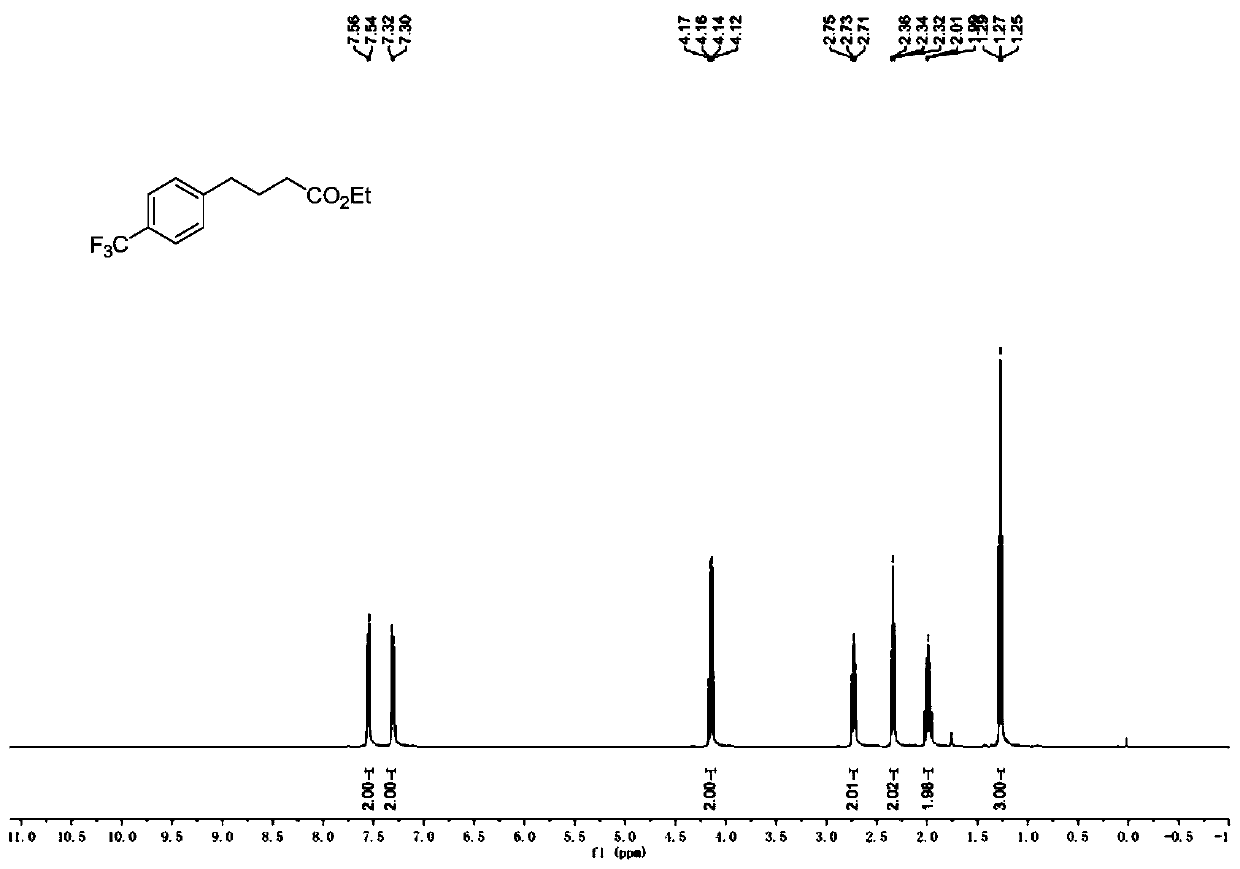
[0083] Add Hans ester Et-HE (0.8mmol), Ru (bpy) to the reaction tube equipped with a magnetic stirrer 3 Cl 2 ·6H 2 O (0.004mmol) and p-thiocresol (0.08mmol). Cover the reaction tube, replace the nitrogen three times, inject 4-trifluoromethylstyrene (0.4mmol), ethyl diazoacetate (0.8mmol) and anhydrous dichloromethane (2.0mL, 0.2M ). Then place the reaction tube at a distance of about 15 cm from a 50W blue light source for illumination and stirring. The reaction was carried out at 25° C. for 12 hours until the diazo was completely consumed (monitored by TLC). After removing the solvent by rotary evaporation under reduced pressure, the crude product was obtained. The crude product was purified by silica gel column chromatography to obtain the corresponding product (petroleum ether:ethyl acetate=30:1), the total yield: 81%.
[0084] 1 H NMR (400MHz, CDCl 3 )δ7.55(d, J=8.0Hz, 2H), 7.31(d, J=8.0Hz, 2H), 4.15(q, J=7.1Hz, 2H), 2.73(t, J=7.7Hz, 2H) ,2.34(t,J=7.4Hz...
Embodiment 3
[0086]
[0087] Add Hans ester Et-HE (0.8mmol), Ru (bpy) to the reaction tube equipped with a magnetic stirrer 3 Cl 2 ·6H 2 O (0.004mmol) and p-thiocresol (0.08mmol). The reaction tube was capped, and after the nitrogen had been replaced three times, 4-cyanostyrene (0.4 mmol), ethyl diazoacetate (0.8 mmol) and anhydrous dichloromethane (2.0 mL, 0.2 M) were injected into the reaction tube with a micro syringe. Then place the reaction tube at a distance of about 15 cm from a 50W blue light source for illumination and stirring. The reaction was carried out at 25° C. for 12 hours until the diazo was completely consumed (monitored by TLC). After removing the solvent by rotary evaporation under reduced pressure, the crude product was obtained. The crude product was purified by silica gel column chromatography to obtain the corresponding product (petroleum ether:ethyl acetate=25:1), the total yield: 86%.
[0088] 1 H NMR (400MHz, CDCl 3 )δ7.59(d, J=7.9Hz, 2H), 7.30(d, J=7.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com