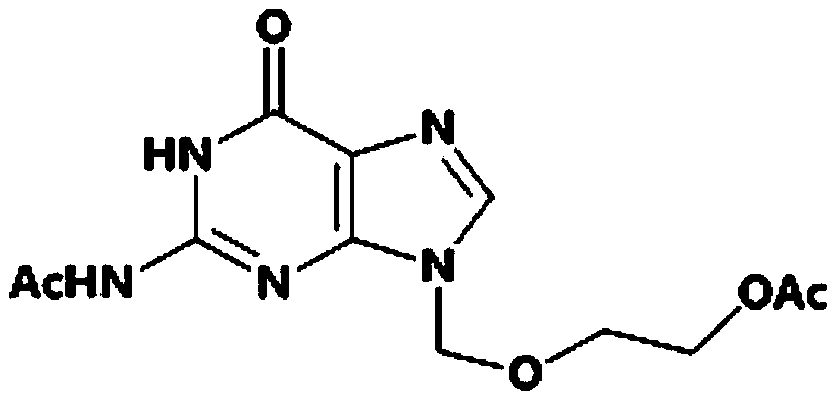
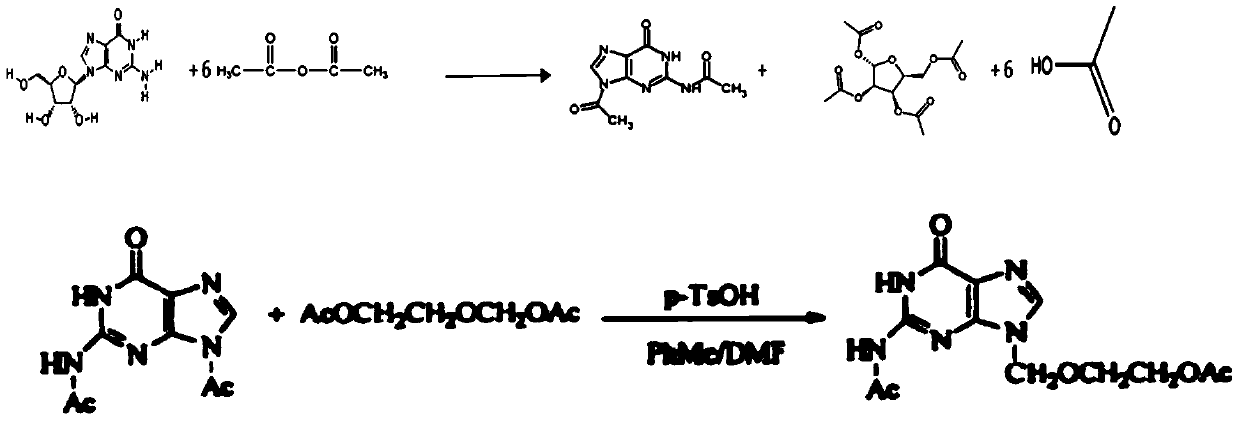
Method for synthesizing diacetyl acyclovir by utilizing guanosine
A technology of diacetyl acyclovir and diacetyl guanine, which is applied in the field of synthesizing diacetyl acyclovir from guanosine, can solve problems such as overflow of reaction materials, improve safety, reduce processing costs, and reduce solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) uniformly mix a part of acetic anhydride and guanosine to obtain a mixed solution 1; uniformly mix another part of acetic anhydride (equal to the acetic anhydride in the mixed solution 1) and boric acid, and heat up to 120° C. to obtain a mixed solution 2; Slowly add the mixed solution 1 to the mixed solution 2 at a rate of 5 cubic meters per hour, and keep it warm at 120°C for 6 hours after the addition, wherein the total amount of acetic anhydride in the guanosine, mixed solution 1 and mixed solution 2 The mass ratio of boric acid to boric acid is 1:6.6:0.008; then distill part of the acetic acid under the condition of slight negative pressure below 0.04MPA, the amount of distilled acetic acid is 25% of the total amount of acetic acid produced in theory, after the distillation is completed, keep warm at 120°C to continue React for 6.5 hours; after the reaction is completed, cool down to 4°C and filter or suction filter to collect the filter cake and filtrate; the f...
Embodiment 2
[0047] (1) uniformly mix a part of acetic anhydride and guanosine to obtain a mixed solution 1; uniformly mix another part of acetic anhydride (equal to the acetic anhydride in the mixed solution 1) and boric acid, and heat up to 125° C. to obtain a mixed solution 2; Slowly add the mixed solution 1 to the mixed solution 2 at a rate of 4 cubic meters per hour, and keep it warm at 125°C for 6 hours after the addition, wherein the total amount of acetic anhydride in the guanosine, mixed solution 1 and mixed solution 2 The mass ratio to boric acid is 1:6.6:0.008; then distill part of the acetic acid under the condition of a slight negative pressure below 0.04MPA, and the amount of distilled acetic acid is 20% of the total amount of acetic acid produced in theory. React for 6 hours; after the reaction is completed, cool down to 0°C and filter or suction filter to collect the filter cake and filtrate; the filter cake is further washed twice with acetic anhydride and dried in vacuum t...
Embodiment 3
[0053] (1) uniformly mix a part of acetic anhydride and guanosine to obtain a mixed solution 1; uniformly mix another part of acetic anhydride (equal to the acetic anhydride in the mixed solution 1) and boric acid, and heat up to 120° C. to obtain a mixed solution 2; Slowly add the mixed solution 1 to the mixed solution 2 at a rate of 6 cubic meters per hour. After the addition, keep the temperature at 120°C for 6.5 hours, wherein the total amount of guanosine, mixed solution 1 and mixed solution 2 is acetic anhydride. The mass ratio of boric acid to boric acid is 1:6.6:0.008; then part of the acetic acid is distilled under the condition of a slight negative pressure below 0.04MPA, and the amount of distilled acetic acid is 30% of the total amount of acetic acid produced in theory. After the distillation is completed, it is kept at 120°C Continue the reaction for 7 hours; after the reaction is completed, cool down to 5°C and filter or suction filter to collect the filter cake a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com