Method for improving recombinant human albumin expression quality and reducing degradation and application
A technology for human albumin and mass reduction, applied in the field of genes, can solve problems such as affecting the quality of recombinant proteins in purification work, and achieve the effect of reducing wrong shearing and improving protein quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1 Construction of engineering bacteria containing only human albumin gene
[0133] Recombinant Engineering Bacteria I
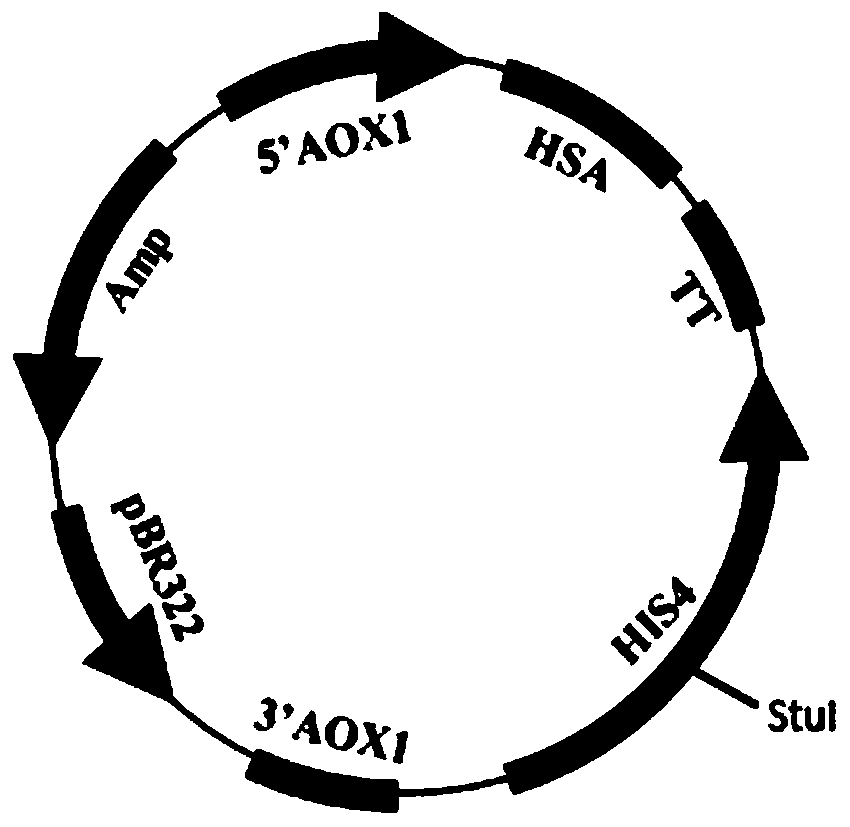
[0134] The transformed recombinant plasmid is pPic9-HSA, and its structure diagram is shown in figure 1 shown. The human albumin signal peptide coding sequence is shown in SEQ ID NO.1, and the human albumin mature peptide coding sequence is shown in SEQ ID NO.4.
Embodiment 2
[0135] Example 2 Construction of Engineering Bacteria Co-expressing Yeast Protein Disulfide Bond Isomerase
[0136] Recombinant Engineering Bacteria II
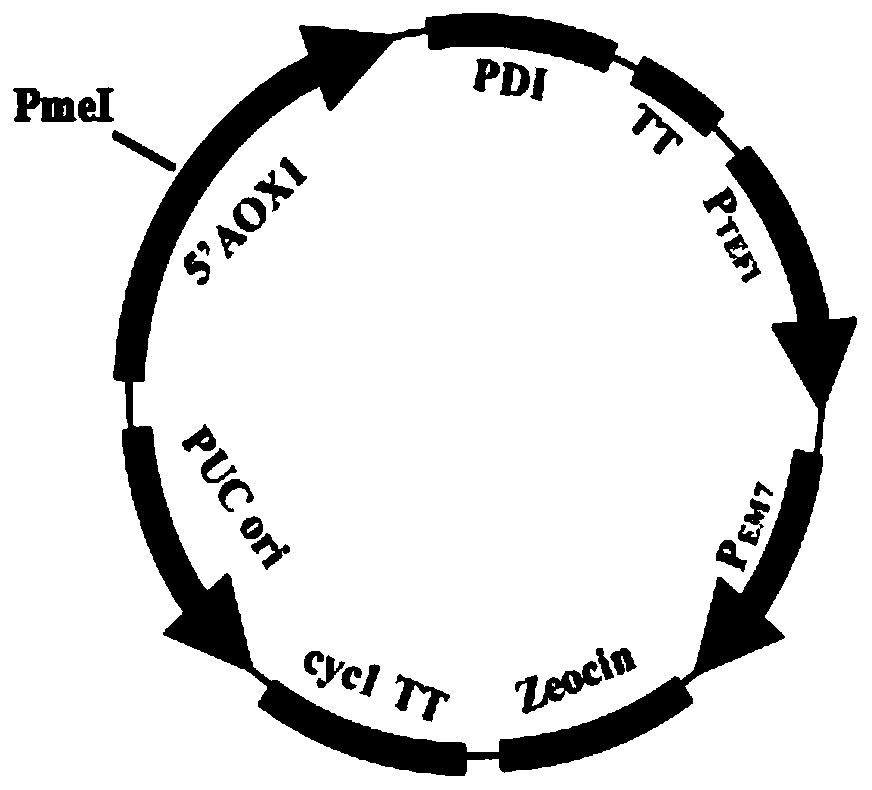
[0137] Respectively pPicZA-PDI (structural schematic diagram as figure 2 shown) and pPic9-HSA plasmid were recombined into the KM71 cell genome twice, the human albumin signal peptide coding sequence is shown in SEQ ID NO.1, and the human albumin mature peptide coding sequence is shown in SEQ ID NO.3. The signal peptide coding sequence of yeast protein disulfide bond isomerase is shown in SEQ ID NO.6, and the mature peptide coding sequence is shown in SEQ ID NO.7.
[0138] Recombinant Engineering Bacteria III
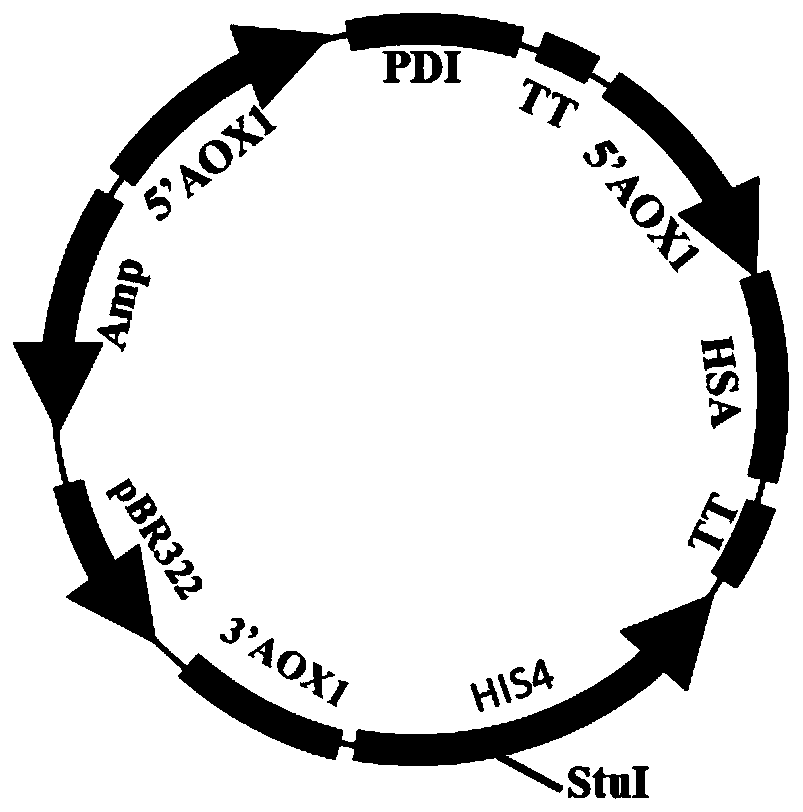
[0139] Respectively pPic9-HSA-PDI (structural schematic diagram as image 3 shown) and pPicZA-HSA-PDI (structural schematic diagram as Figure 4 Shown) the plasmid was recombined into the KM71 cell genome twice, the human albumin signal peptide coding sequence is shown in SEQ ID NO.1, and the human albumin mature ...
Embodiment 3
[0140] Example 3 Construction of Engineering Bacteria Co-expressing Human Protein Disulfide Bond Isomerase
[0141] Recombinant Engineering Bacteria IV
[0142] The pPicZA-PDI and pPic9-HSA plasmids were recombined into the KM71 cell genome twice, the human albumin signal peptide coding sequence is shown in SEQ ID NO.1, and the human albumin mature peptide coding sequence is shown in SEQ ID NO.3. Show. The signal peptide coding sequence of human protein disulfide bond isomerase is shown in SEQ ID NO.9, and the mature peptide coding sequence is shown in SEQ ID NO.11.
[0143] Recombinant engineered bacteria V
[0144] The transformed recombinant plasmid is pPic9-HSA-PDI, and the structure diagram is as follows image 3 shown. The human albumin signal peptide coding sequence is shown in SEQ ID NO.1, and the human albumin mature peptide coding sequence is shown in SEQ ID NO.3. The signal peptide coding sequence of human protein disulfide bond isomerase is shown in SEQ ID NO....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com