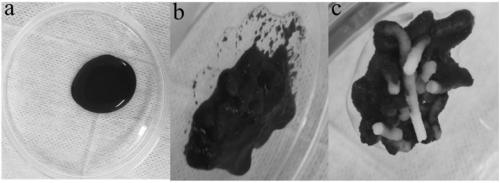
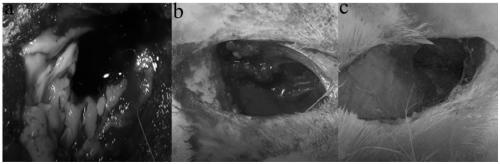
Fluid haemostatic adhesive and preparation method thereof
A fluid and composite gel technology, applied in the medical field, can solve problems such as poor adhesion, unsuitable wounds with high blood flow, and no fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1: the preparation of cross-linked carboxymethyl chitosan
[0089] Weigh 0.8g sodium hydroxide, dissolve in 185mL water to obtain 0.1mol / L sodium hydroxide solution; weigh 15.0g carboxymethyl chitosan (deacetylation degree 90.1%, molecular weight 20kDa), dissolve in the above-mentioned hydroxide In the sodium solution, stir for 1 h to obtain a carboxymethyl chitosan solution with a mass fraction of 7.5%; add 0.075 g of 1,4-butanediol diglycidyl ether to the carboxymethyl chitosan solution, and stir for 0.5 h; Adjust the reaction temperature to 20-60°C, and let it stand for 20 hours to obtain cross-linked carboxymethyl chitosan; add 200 mL of 0.1mol / L hydrochloric acid solution to the cross-linked carboxymethyl chitosan to carry out acid-base neutralization reaction, And use 0.1mol / L hydrochloric acid solution to adjust the pH value of cross-linked carboxymethyl chitosan to about 6.8-7.6; transfer the neutralized cross-linked carboxymethyl chitosan to a homoge...
Embodiment 2
[0090] Embodiment 2: the preparation of cross-linked carboxymethyl chitosan
[0091] Weigh 0.8g sodium hydroxide, dissolve in 185mL water to obtain 0.1mol / L sodium hydroxide solution; weigh 15.0g carboxymethyl chitosan (deacetylation degree 90.1%, molecular weight 20kDa), dissolve in the above-mentioned hydroxide In the sodium solution, stir for 1 h to obtain a carboxymethyl chitosan solution with a mass fraction of 7.5%; add 0.15 g of 1,4-butanediol diglycidyl ether to the carboxymethyl chitosan solution, and stir for 0.5 h; Adjust the reaction temperature to 20-60°C, and let it stand for 20 hours to obtain cross-linked carboxymethyl chitosan; add 200 mL of 0.1mol / L hydrochloric acid solution to the cross-linked carboxymethyl chitosan to carry out acid-base neutralization reaction, And use 0.1mol / L hydrochloric acid solution to adjust the pH value of cross-linked carboxymethyl chitosan to about 6.8-7.6; transfer the neutralized cross-linked carboxymethyl chitosan to a homogen...
Embodiment 3
[0092] Embodiment 3: the preparation of cross-linked gelatin
[0093] Weigh 30.0 g of gelatin particles (particle size is 500 μm, pH is 5.5), dissolve in 570 mL of water, stir for 0.5 h to obtain a gelatin solution with a mass fraction of 5%; adjust the pH of the system to 5.0 with 0.1 mol / L hydrochloric acid solution ~5.5, add 0.3 g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride to the gelatin aqueous solution, and continue to use 0.1mol / L hydrochloric acid solution to adjust the pH value of the system to 5.0~ 5.5; Adjust the reaction temperature to 2-10°C and react for 15 hours under stirring to obtain cross-linked gelatin; transfer the reaction solution to a 3L beaker, add 2L of water, react for 30min under stirring, then let stand for 30min, discard the supernatant solution, repeat the above cleaning operation more than 15 times; transfer the gelatin solution to the VFD-1000 freeze dryer of Beijing Boyikang Experimental Instrument Co., Ltd., and freeze-dry for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com