Immune effector cell conversion receptor
A technology of immune effector cells and receptors, which is applied in the fields of medical immunology and molecular biology, and can solve the problems of poor effector effect, unsatisfactory killing effect of target cells, short proliferation and duration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0132]The method for preparing cells expressing immune effector cell switching receptors of the present invention includes the step of introducing the nucleic acid encoding immune effector receptors of the present invention into cells. This step can be performed ex vivo. For example, cells expressing the immune effector cell receptors of the present invention can be prepared in vitro by transducing viral vectors or non-viral vectors containing the nucleic acids of the present invention.
[0133] In the preparation method of the present invention, cells from mammals, such as cells derived from humans, or cells derived from non-human mammals, such as monkeys, mice, rats, pigs, horses, cows, etc., can be used. , sheep or dog cells.
[0134] In one embodiment, the mammal is a human.
[0135] The cells used in the preparation method of the present invention are not particularly limited, and any cells can be used. For example, cells collected, isolated or purified from bodily flu...
Embodiment 1
[0164] Example 1 Construction of expression vectors expressing EGFR switching receptors
[0165] 1. Entrust Shanghai Jierui Biological Company to synthesize the EGFP extracellular region gene, introduce a polyclonal restriction site (BglII-XbaI-EcoRI-BamHI) upstream of it, and insert a restriction site (SalI-NheI-HindIII- SpeI), which was loaded into the pNB328 vector cut with EcoR1+SalI (see CN201510638974.7 for the structure and sequence of pNB328, the entire content of which is incorporated herein by reference), to obtain the pNB328-EGFP vector
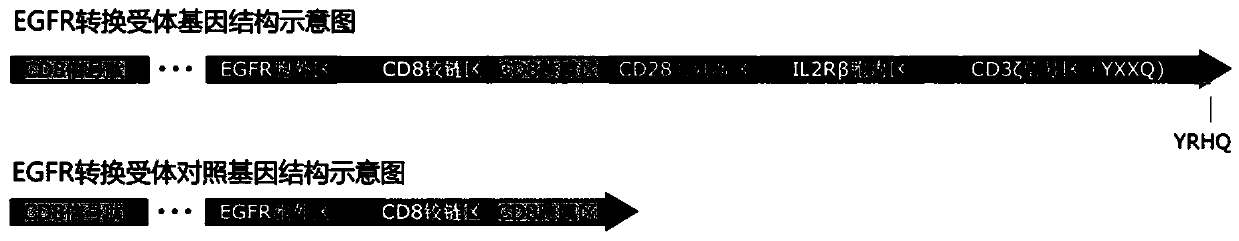
[0166] 2. Entrust Shanghai Jierui Biological Co., Ltd. to synthesize the EGFR switch receptor gene, which sequentially includes CD8 signal peptide, EGFR extracellular region, CD8 hinge region, CD8 transmembrane region, CD28 co-stimulatory region, and truncated IL-2Rβ chain intracellular region and the CD3ζ signal region containing the YRHQ motif, the schematic diagram of which is shown in figure 1 As shown, the sequence is shown i...
Embodiment 2
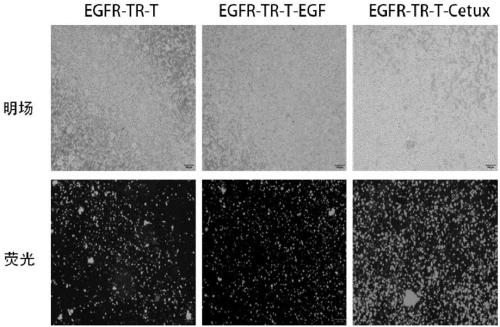
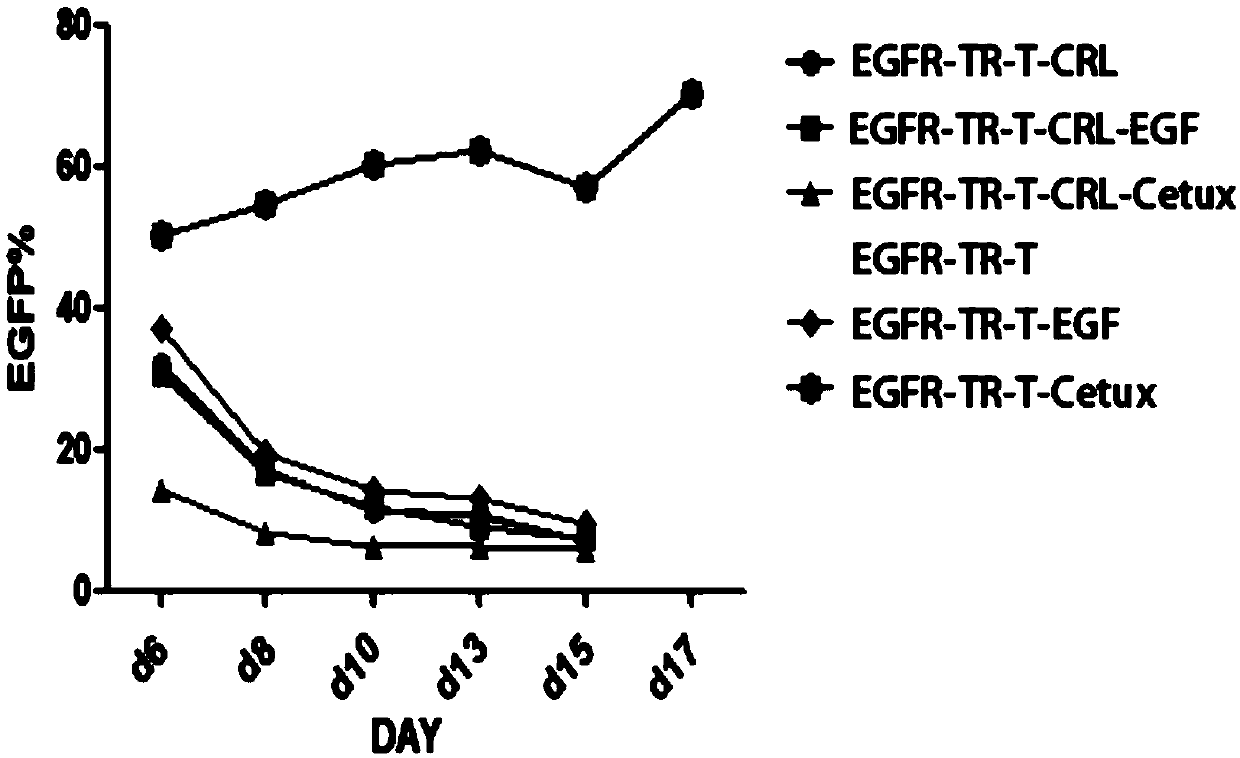
[0169] Example 2 Using Cetuximab and CD28 Monoclonal Antibody to Stimulate T Cells Expressing EGFR Switching Receptor
[0170] 1) Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of donor subjects by Ficoll separation method. Cultivate PBMCs for 2-4 hours. The unattached suspension cells are initial T cells. Collect the suspension cells into a 15ml centrifuge tube, centrifuge at 1200rmp for 3min, discard the supernatant, add physiological saline, centrifuge at 1200rmp for 3min, discard the physiological brine, and repeat this step.
[0171] 2) Take two 1.5ml centrifuge tubes, numbered a and b, and add 5×10 6 Step 1) Centrifuge the initial T cells obtained at 1200rpm for 3min, discard the supernatant, add normal saline, centrifuge at 1200rpm for 3min, discard the normal saline, and repeat this step;
[0172] 3) Coat the six-well plate with a coating solution containing 5 μg / mL cetuximab (purchased from Merck) and 5 μg / mL anti-CD28 antibody (purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com