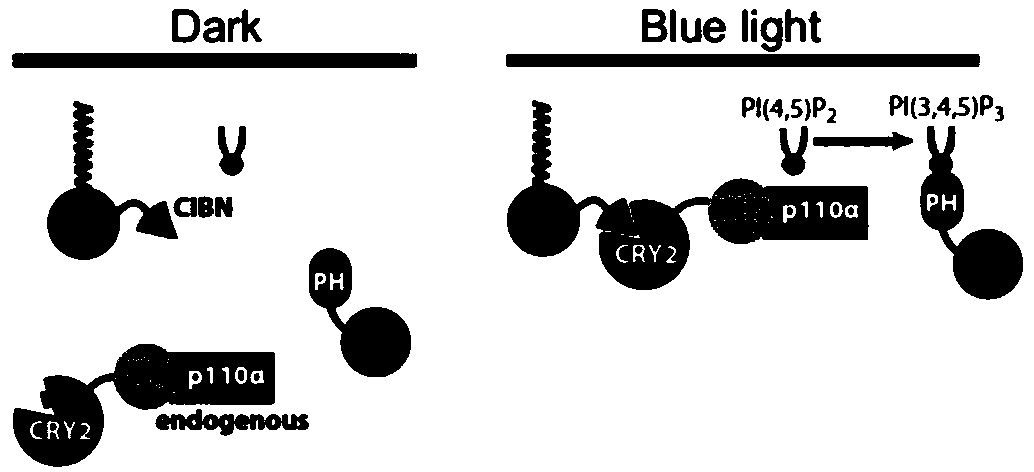
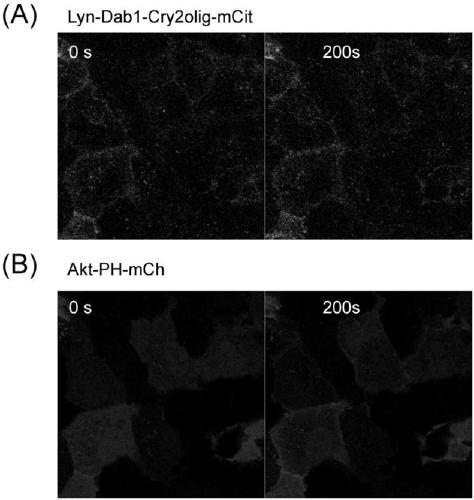
Fusion protein and application thereof
A fusion protein and protein technology, which is applied in the field of biomedicine, can solve the problems of the influence of induction results, time-consuming, unfavorable PI3K/Akt pathway control, etc., and achieve the effect of rapid activation and regulation and high spatial resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
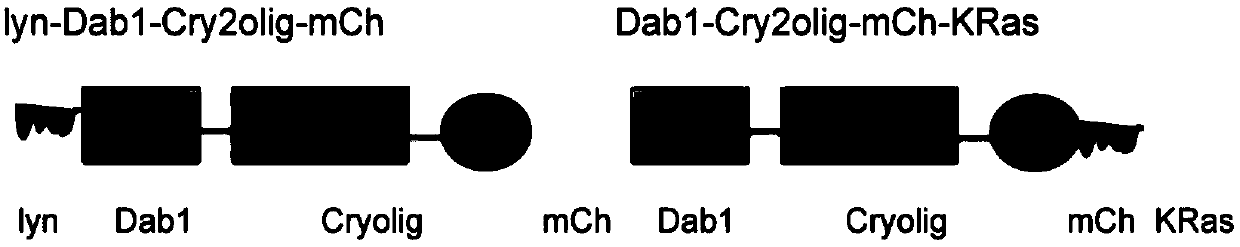
[0041] Preparation of embodiment 1lyn-Dab1-Cry2olig-mCh fusion protein
[0042] Using the basic technology of molecular cloning, the DNA sequences encoding the membrane localization sequence of lyn protein, Dab1 protein, light-sensitive protein Cry2olig and red fluorescent protein mCh (mCherry) were connected, introduced into the expression vector pCAG-DsRed2, and the recombinant expression vector was obtained and transformed into Host bacteria Escherichia coli, induced expression to obtain the fusion protein (see the schematic diagram of the structure figure 2 ), has the amino acid sequence shown in SEQ ID NO:3, and the nucleotide sequence is shown in SEQ ID NO.6.
Embodiment 2
[0043] Example 2 Preparation of lyn-Dab1-Cry2olig-mCit fusion protein
[0044] Using the basic technology of molecular cloning, the DNA sequences encoding the membrane localization sequence of lyn protein, Dab1 protein, light-sensitive protein Cry2olig and yellow fluorescent protein mCit (mCit) were connected, and introduced into the expression vector pCAG-DsRed2 to obtain the recombinant expression vector, which was transformed into The host strain Escherichia coli is induced to express the fusion protein, which has the amino acid sequence shown in SEQ ID NO:4 and the nucleotide sequence shown in SEQ ID NO.7.
Embodiment 3Dab
[0045] Example 3 Preparation of Dab1-Cry2olig-mCit-KRas fusion protein
[0046] Using the basic technology of molecular cloning, the DNA sequences encoding Dab1 protein, light-sensitive protein Cry2olig, yellow fluorescent protein mCit (mCit) and KRas protein membrane localization sequence were connected and introduced into the expression vector pCAG-DsRed2 to obtain a recombinant expression vector, which was transformed into The host strain Escherichia coli is induced to express the fusion protein, which has the amino acid sequence shown in SEQ ID NO:5 and the nucleotide sequence shown in SEQ ID NO.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com