Amidase and coding gene, recombinant carrier, recombinant strain and application thereof
A recombinant vector and amidase technology, applied in the field of enzyme engineering, can solve the problems of low degradation efficiency of ochratoxin and unsuitability for industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: cDNA synthesis and cloning of amidase gene
[0040] The strain is derived from the Stenotrophomonas bacteria obtained in the laboratory in the early stage. Through gene sequence determination, the open reading frame nucleotide sequence of the amidase gene was analyzed, and the upstream primer (adh117-F )(SEQ ID NO.3): 5'-CGC GGATCC ATGCCGATCCGCCGCCGC-3'; downstream primer (adh117-R) (SEQ ID NO.4): 5'-CCG CTCGAG TCACTGCTTGTAGATCACCCG-3', respectively introduce restriction endonuclease sites on the upstream and downstream primers (depending on the carrier selected, BamHI and XhoI enzyme cutting sites have been added in the present invention, underlined, italic sequence It is the homologous sequence at the downstream end of the pGEX-4T-1 vector). Underlined, the sequence in italics is the homologous sequence at the downstream end of the pGEX-4T-1 vector), the amidase gene encoding the amino acid shown in SEQ ID NO.1 was obtained by in vitro amplification...
Embodiment 2
[0041] Example 2: Heterologous Expression of Stenotrophomonas Amidase Gene
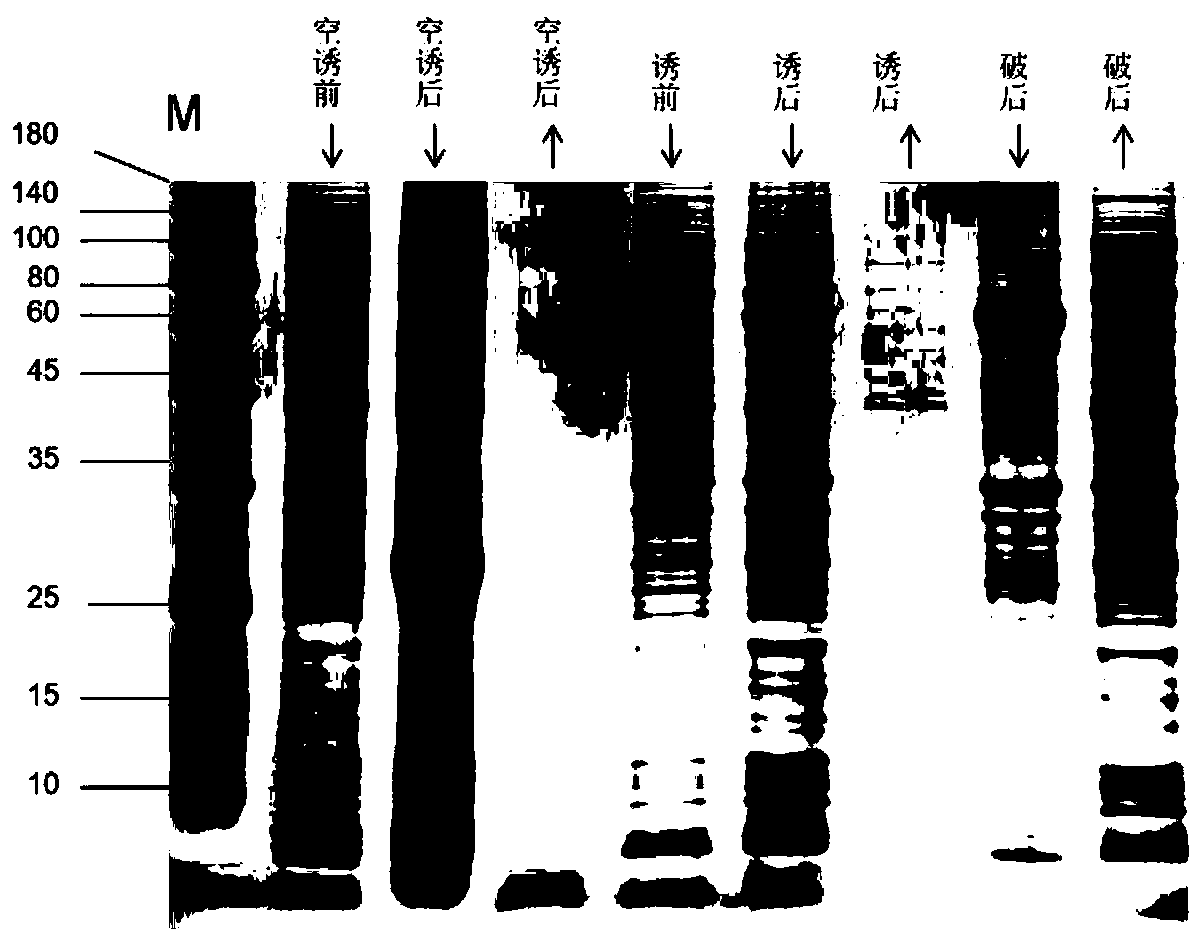
[0042] The E. Coil BL21(DE3) / pGEX-4T-1 / adh117 transformant obtained in Example 1 was shaken overnight in 100 mL LB liquid medium containing 100 μg / mL ampicillin. Draw 1.0 mL of seed bacteria liquid and inoculate it into fresh 100 mL of LB liquid medium (containing 100 μg / mL ampicillin), and culture at 37° C. with shaking at 180 r / min. When the bacteria solution OD 600 When it reaches 0.6, add 0.2mmol / mL IPTG to induce expression at 16°C for 4h. Refrigerate and centrifuge at 7000r / min for 10min, discard the supernatant. Suspend the cells with 10 mL of 1×phosphate buffered saline (PBS), and disrupt the cells by ultrasonic in an ice bath. Centrifuge and refold the pellet for inclusion body renaturation. The results of SDS-PAGE electrophoresis show that the enzyme is present in the supernatant and the precipitate (inclusion body) after the bacterial cell is broken, and its apparent molecular weight (i...
Embodiment 3
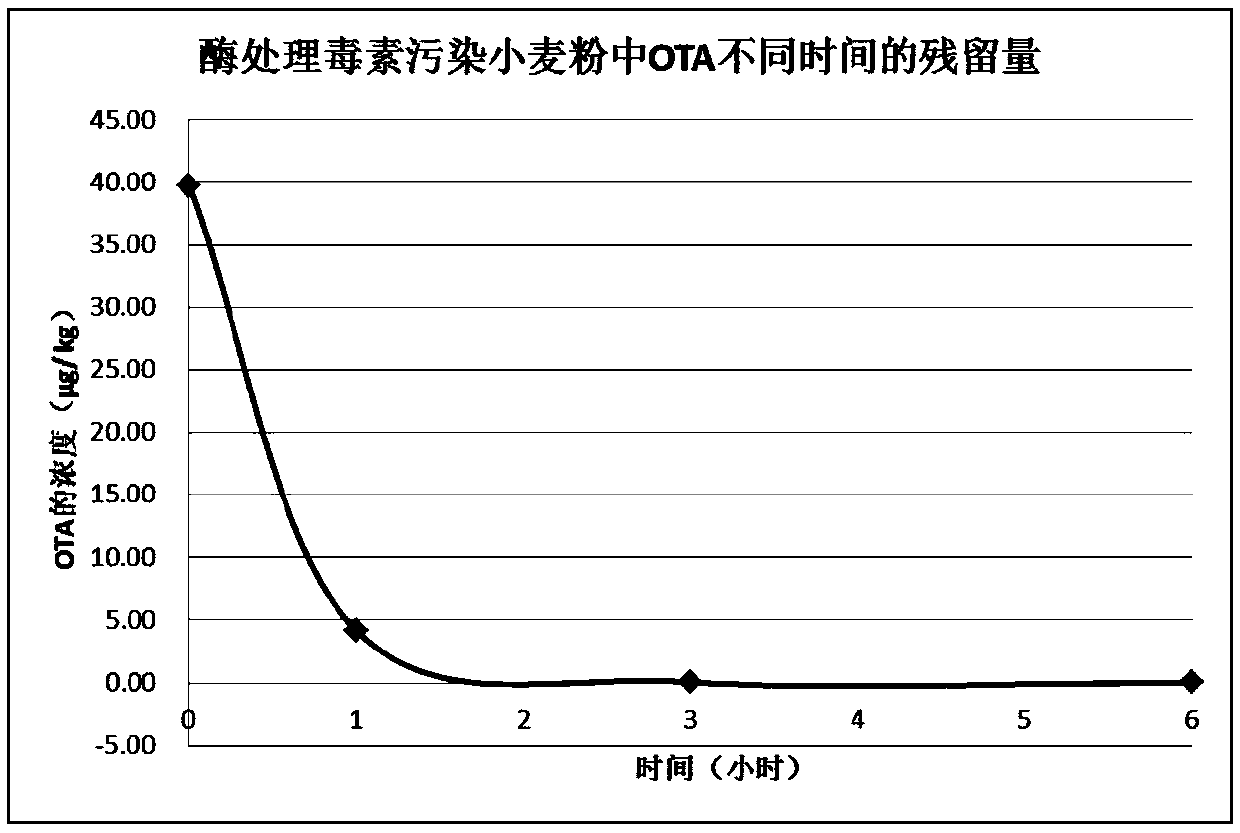
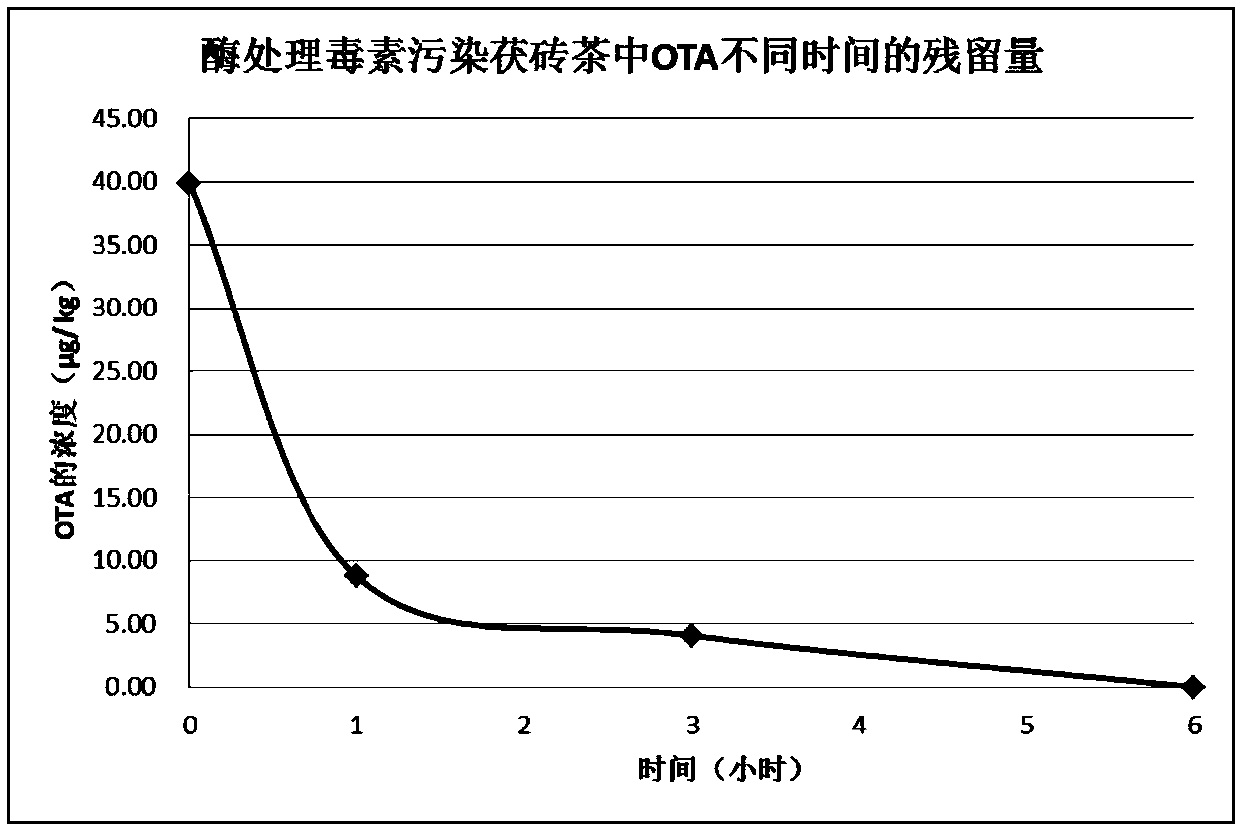
[0043] Example 3: Application of Heterologous Recombination Detoxification Enzyme in Ochratoxin Degradation
[0044] 1. Experimental materials
[0045] The enzyme preparation is the crude enzyme solution of the crushed supernatant after the expression of the pGEX-4T-1 / adh239 transformant, and other reagents are analytically pure chemical reagents.
[0046] 2. Experimental method
[0047] Take 10 μL of the crushed supernatant after expression of the pGEX-4T-1 / adh117 transformant and put it in a 2 mL centrifuge tube, add it to 490 μL of ochratoxin A standard buffer (use 1*PBS to prepare pH 7.3), ochratoxin A The final concentration of the solution is about 40 μg / L, react for 2 minutes, and add 1.5 mL of acetonitrile to the reaction system to stop the reaction. High performance liquid chromatography was used to detect the residual amount of OTA. The experimental results showed that after 2 minutes of reaction, the broken supernatant degraded all OTA.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com