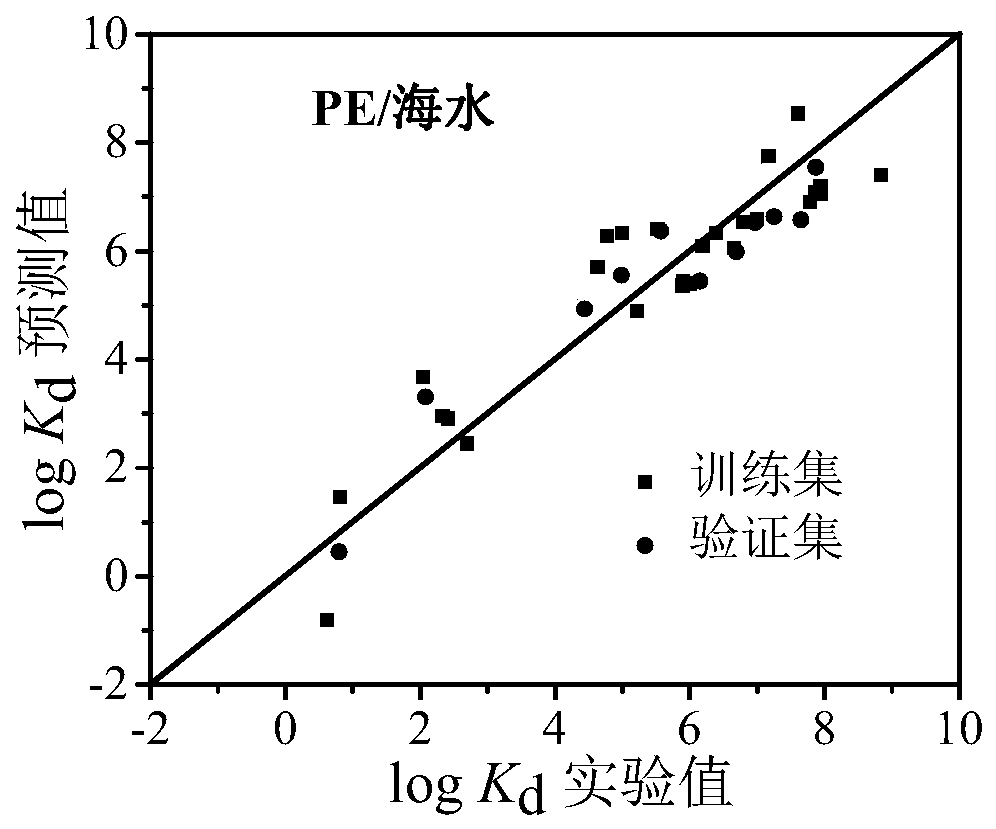
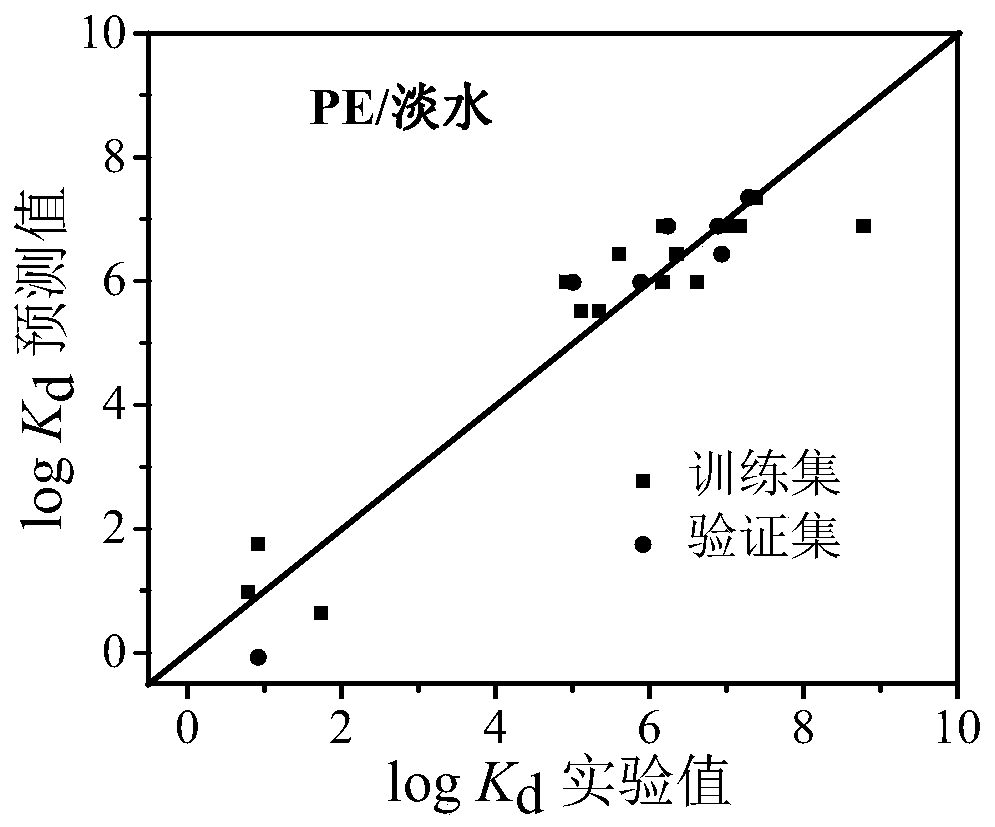
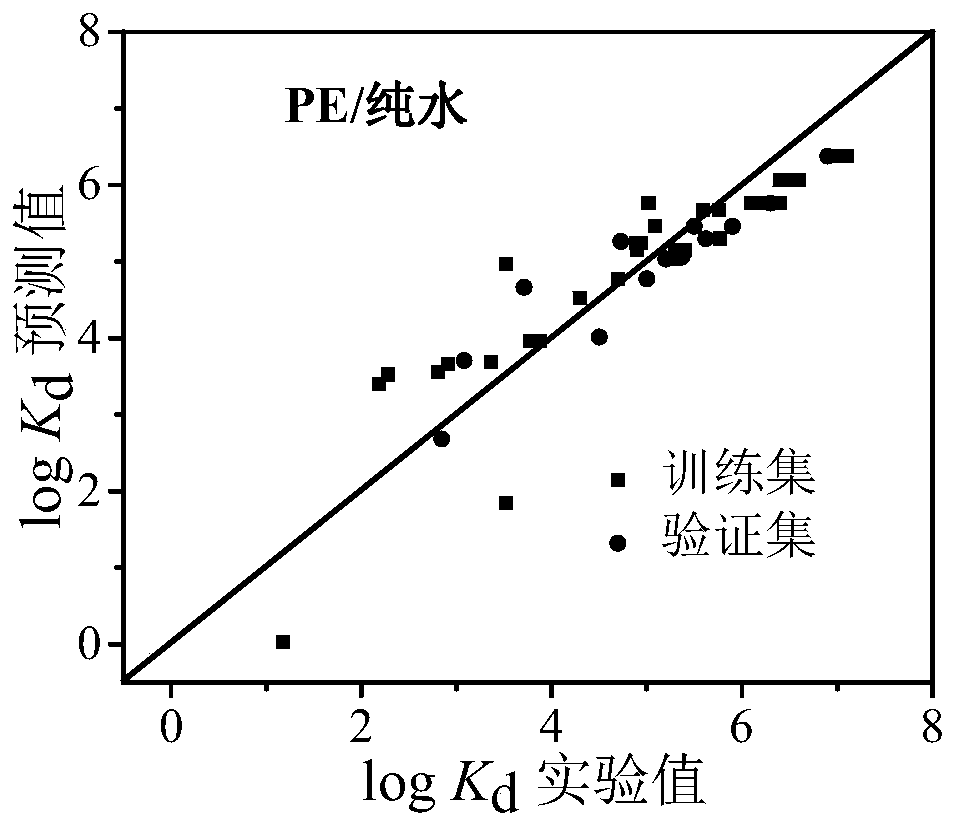
Method for predicting distribution equilibrium constant of organic pollutants between polyethylene type micro-plastics and water phase
A technology of organic pollutants and distribution balance, applied in chemical property prediction, chemical machine learning, chemical data mining, etc., can solve problems such as model verification and representation, model parameters dependent on experimental determination, and the number of model compounds is small, and achieve practical application capabilities Strong, simple and easy regression analysis, wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 perfluorooctanoic acid (PE / seawater)
[0047] In this example, the log K of perfluorooctanoic acid between PE / seawater d The value is predicted, and its h is calculated by using the Williams graph method i The value is 0.195α and ε β The values are 0.31 and 0.30, respectively. Using ACD Labs 6.0 software to calculate, the log D value of the compound was 4.00.
[0048] Bring the above descriptors into model(1):
[0049] log K d =0.725×log D-23.169×ε β -36.236×ε α +17.856
[0050] =0.725×4.00-23.169×0.30-36.236×0.31+17.856
[0051] =2.57
[0052] According to the model (1), the log K of perfluorooctanoic acid was obtained d The predicted value is 2.57, and its log K in the literature d The value is 2.70, and the predicted value is in good agreement with the experimental value.
Embodiment 2
[0053] Example 2 2,3,3',4,4'-pentachlorobiphenyl (PE / fresh water)
[0054] In this example, the log K between PE / fresh water for 2,3,3',4,4'-pentachlorobiphenyl d The value is predicted, and its h is calculated by using the Williams graph method i The value is 0.048-3, indicating that this compound is within the application domain of the QSPR model. Calculated by ACD Labs 6.0 software, the log D value of the compound was 6.98.
[0055] Bring the above descriptors into model(2):
[0056] log K d =0.667×log D+1.714
[0057] =0.667×6.98+1.714
[0058] =6.37
[0059] The log K of 2,3,3',4,4'-pentachlorobiphenyl was obtained according to the model (2) d The predicted value is 6.37, and its log K in the literature d The value is 6.35, and the predicted value is in good agreement with the experimental value.
Embodiment 3
[0060] Embodiment 3 cyclohexane (PE / pure water)
[0061] This embodiment is to the log K of cyclohexane between PE / pure water d The value is predicted, and its h is calculated by using the Williams graph method i The value is 0.012<h*(warning value)=0.13, standard residual (SE)=0.539<3, indicating that this compound is within the application domain of the QSPR model. Calculated by ACD Labs 6.0 software, the log D value of the compound was 3.18.
[0062] Bring the above descriptors into model(3):
[0063] log K d =0.486×log D+2.420
[0064] =0.486×3.18+2.420
[0065] =3.97
[0066] According to the model (3), the log K of cyclohexane is obtained d The predicted value is 3.97, and its log K in the literature d The value is 3.88, and the predicted value is very consistent with the experimental value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com