Cyclooxygenase targeted near infrared dye metal complex photosensitizer and preparation and application thereof
A near-infrared dye and metal complex technology, applied in the field of near-infrared dye metal complex photosensitizers, can solve problems such as limited selectivity, and achieve the effects of convenient synthesis, good water solubility, and stable construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] Further, the present invention also provides a method for preparing the above-mentioned metal complex photosensitizer, including:
[0111] Will (M) p (L 1 ) q (X 6 ) r with L 2 Ligand structure compound (when L2 is a neutral ligand, then directly with compound L 2 response when L 2 When it is an anionic ligand, it generally contains L 2 Compounds as raw materials, such as HL 2 etc.) reaction to obtain cyclooxygenase-targeted near-infrared dye metal complex photosensitizer;
[0112] Among them, M, L 1 , L 2 The definition of can refer to the relevant structures and definitions in the above formulas (I), (II), and (III);
[0113] At the same time, p and q are integers greater than or equal to 1; when q is greater than 1, different L 1 may independently be the same or different groups;
[0114] r is an integer greater than or equal to 0;
[0115] x 6 For the counter ion, the metal complex photosensitizer provided by the present invention can be a neutral met...
Embodiment 1
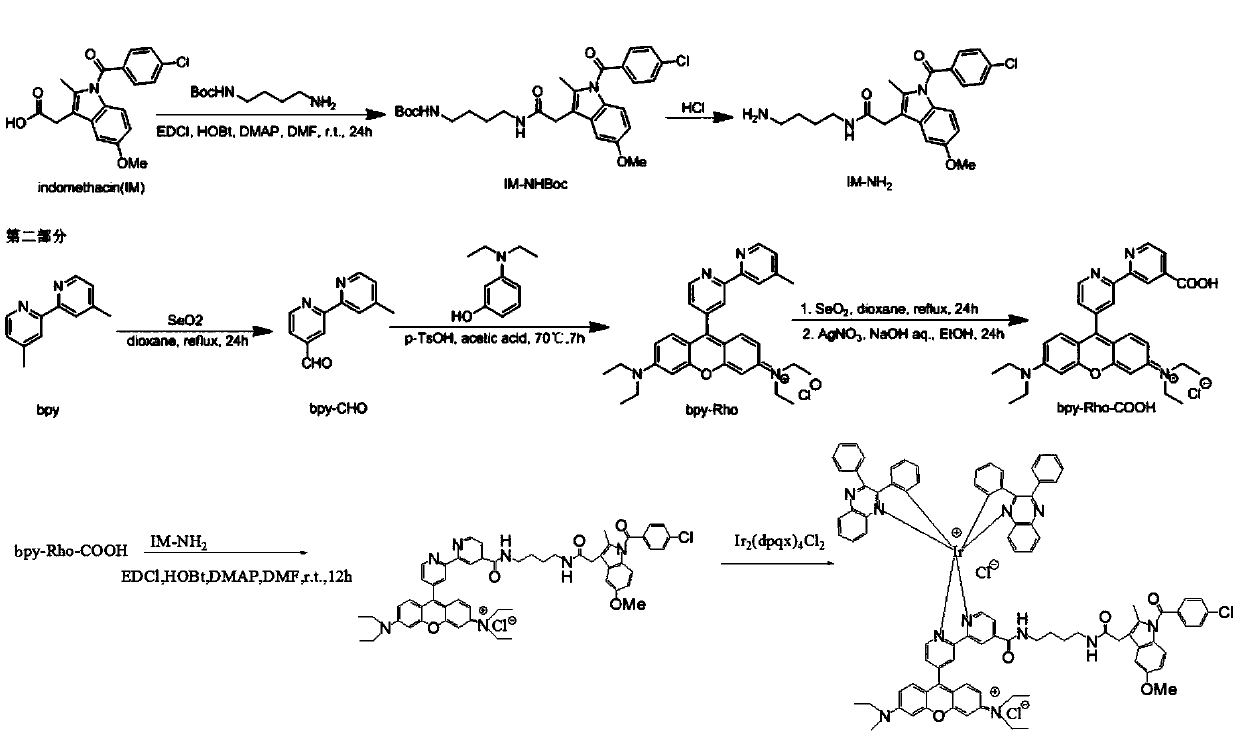
[0159] At room temperature, add indomethacin (100mg) and 1,4-butanediamine-Boc (100mg) and coupling reagents EDCI, HOBt, DMAP to a 25mL round-bottomed flask, and after stirring rapidly for about 0.5 hours, the solution becomes Transparent, the reaction occurs at room temperature for 24 hours, stop the reaction, spin off the solvent, purify with a silica gel column, dissolve the sample in dichloromethane, load the sample by wet method, use dichloromethane:methanol as the eluent from 200:1 to 10:1 Gradient elution, spin off the solvent and concentrate to obtain IM-NHBoc. Yield 80%, IM-NHBoc is deprotected under the effect of hydrochloric acid to obtain IM-NH 2 .
[0160] First, bpy (3.27g) was dissolved in 1,4-dioxane (150ml) and put into a 250ml round bottom flask, then SeO was added at room temperature 2 (2.174g). Put the mixture in N 2 Reflux for 24 hours in a dark environment (mild reflux, 100°C). The mixture was then filtered hot and the solution was cooled to room tem...
Embodiment 2
[0165] With reference to Example 1 method, prepare Rho-IM (rhodamine-indomethacin ligand), then, then add 8mg Ir 2 (ppy) 4 -Cl 2 Add 3ml each of DCM and methanol to a 25ml round bottom flask, reflux at 65°C for 4h, then add 26mg of KPF6 and react at room temperature for 30min to obtain the target product Ir-Rho-IM and then purify.
[0166] Embodiment 2 reaction process is as Figure 7 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com