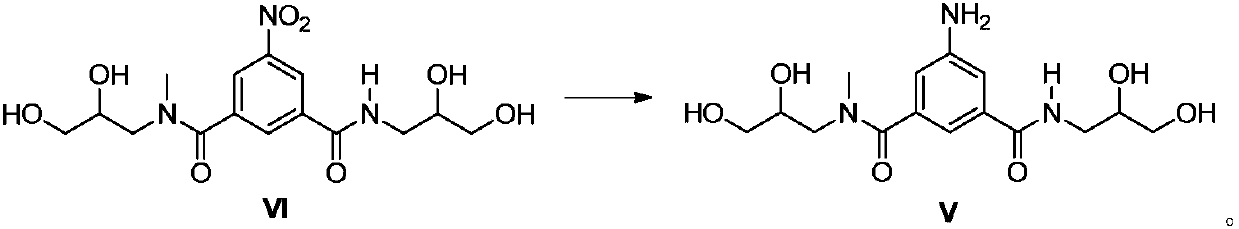
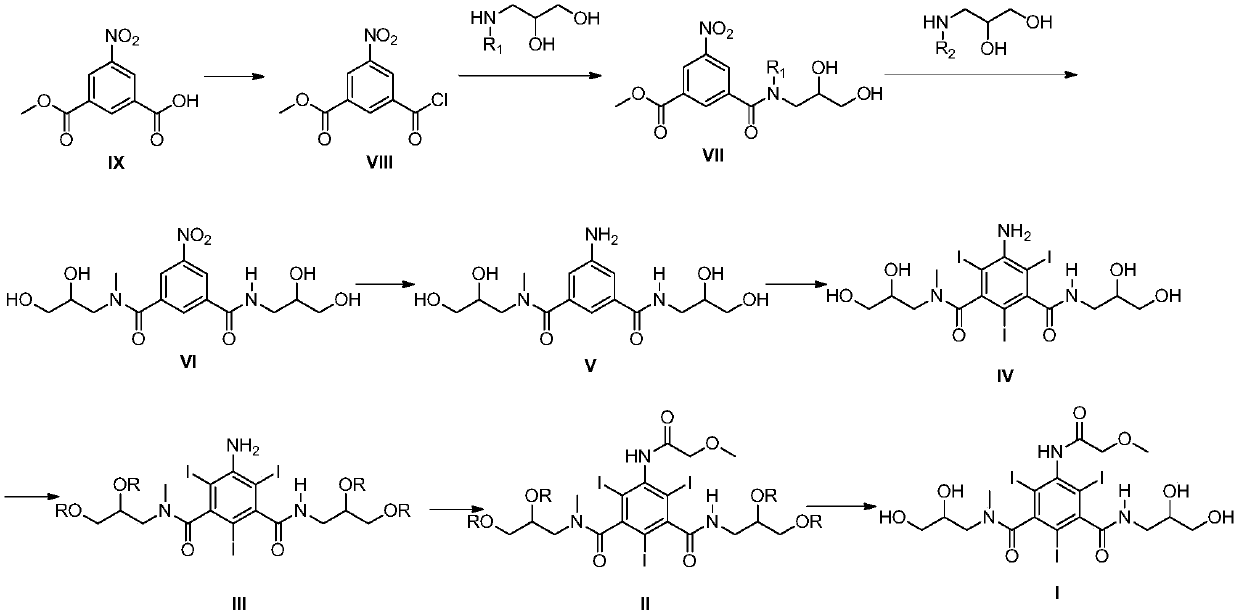
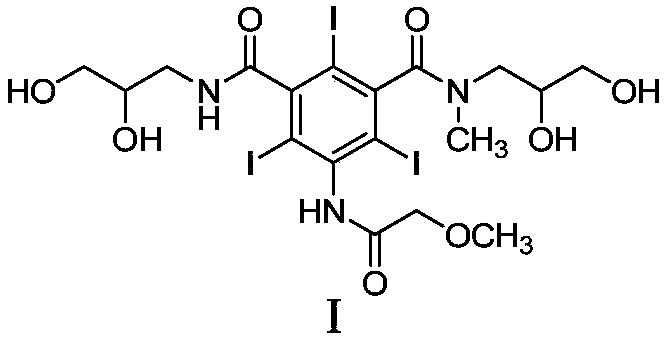
Preparation method of iopromide intermediate and application thereof
A technology of iopromide and compounds, applied in the field of drug synthesis, can solve the problems of many by-products in the iodination reaction step, unfavorable industrial production, and difficult separation and purification, so as to avoid the generation of diacylated by-products and facilitate separation and purification , Good industrial applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of 2-methoxyacetyl chloride
[0057]
[0058] 2-Methoxyacetic acid (656.7mmol), dichloromethane (197mL) and DMF (21.9mmol) were added into a 500mL three-neck flask, the reaction solution was cooled to 10°C, and thionyl chloride (525.3mmol ), dropwise, react at room temperature for 12 hours, and concentrate the reaction solution at 25°C to obtain 2-methoxyacetyl chloride.
Embodiment 2
[0059] Embodiment 2: Preparation of 3-chloroformyl-5-nitrobenzoic acid methyl ester (compound of formula VIII)
[0060]
[0061] Add the compound of formula IX (66.62mmol) into a 250mL three-necked flask, add dichloromethane (45mL) and DMF (0.075mL), heat in a water bath at 25°C, disperse for 10 minutes, slowly add oxalyl chloride (99.9mmol) dropwise, and react After the liquid was clarified, continue to stir for half an hour, then concentrate, add dichloromethane (15mL×2) and continue to concentrate twice to obtain a white solid compound 3-chloroformyl-5-nitrobenzoic acid methyl ester, add di Chloromethane (37 mL) was dissolved for use.
Embodiment 3
[0062] Embodiment 3: Preparation of 3-((2,3-dihydroxypropyl)carbamoyl)-5-nitrobenzoic acid methyl ester (compound of formula VII-1)
[0063]
[0064] Add aminoglycerol (159.9mmol) and absolute ethanol (22.5mL) into a three-neck flask, stir at room temperature to dissolve, then cool the reaction solution to -30°C-20°C, slowly add the formula VIII prepared in Example 2 dropwise For the dichloromethane solution of the compound, the temperature of the feed liquid is controlled not to be higher than -13° C. during the dropwise addition, and the stirring is continued for 0.5 hours after the dropwise addition. After the reaction was finished, 1mol / L dilute hydrochloric acid was added dropwise to adjust the pH to 1-2, stirred for half an hour, warmed up to room temperature, separated, the organic phase was washed with 60mL of water, the aqueous phase was combined, and a mixed solvent of dichloromethane / ethanol was added ( Dichloromethane / ethanol=2:1) (60mL×2) extracted twice, com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com