Construction method of sea bass fry cell line
A technique for constructing method and cell line
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
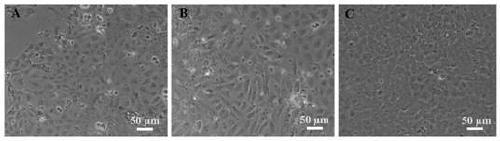
[0031] Embodiment 1: the construction method of sea bass larvae cell line
[0032] (1) Selection of larvae: select sea bass fry hatched for 3-4 days, and the body length of the experimental fish is 5-7mm.
[0033] (2) Preparation of rinse solution and cell culture solution:
[0034] Basal medium: Leibovitz's-15 (L15) medium, M199 medium, Eagle's minimalessential medium (MEM) medium are all products of Gibco. Each medium was prepared according to the company's product instructions, and 0.266% NaCl and 5mM HEPES were added, filtered through a 0.22μm filter membrane, subpackaged, and stored at 4°C for later use; when used, add 10-20% fetal bovine serum, 1-4 times PSN Antibody mixture (penicillin, streptomycin, nystatin).
[0035] Rinsing solution: basal medium L-15 containing 400 IU / mL penicillin, 400 μg / mL streptomycin and 100 μg / mL nystatin, pH 7.2-7.4.
[0036] Primary cell culture medium: basal medium L-15 contains 20% fetal bovine serum, 0.266% NaCl, 5mM HEPES, 400IU / ml p...
Embodiment 2
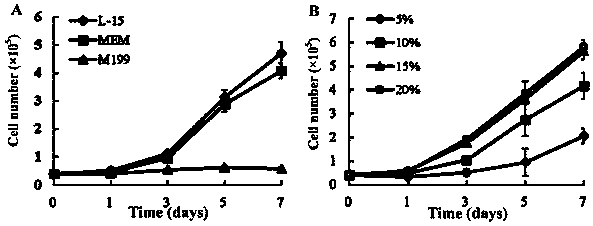
[0048] Embodiment 2 detects the influence of different culture medium and FBS concentration on sea bass larvae cell growth
[0049] 1. The effect of different culture media on cell growth.
[0050] Using the 40th generation seabass larvae cells in Example 1, the growth curves of the cells in different culture media were detected, and the specific operations were as follows: suck out the culture medium in the culture bottle, rinse once with trypsin, and add 2ml of 0.25% Trypsinize the cells until they are completely detached, add medium and gently pipette the cell suspension to separate the cells. Count the cell density with a hemocytometer.
[0051] 0.4×10 respectively 5 Each cell was seeded in a 24-well plate, and the medium was L-15, MEM and M199 medium with 10% fetal bovine serum, respectively, and cultured in a constant temperature incubator at 28°C. The cells were counted with a hemocytometer at 1, 3, 5, and 7 days after culture, and the growth curves of sea bass larva...
Embodiment 3
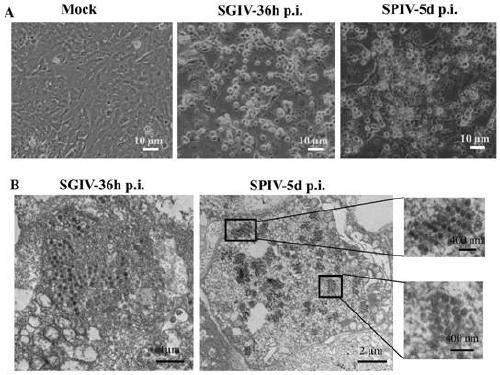
[0057] Example 3 verifies the cryopreservation and resuscitative ability of sea bass larvae cell line cells of the present invention
[0058] 1. Cell cryopreservation
[0059] Take the adherent monolayer cells of passage 35 in Example 1, digest with trypsin to obtain a single cell suspension, centrifuge at 1000 rpm for 10 min, and discard the supernatant. The cell pellet was resuspended with 4°C pre-cooled freezing solution (L-15 medium containing 20% fetal calf serum and 10% DMSO), and 1ml of each tube was transferred to a 2ml cryopreservation tube. The cryopreservation procedure is: 4°C, 30min; -20°C, 2h; -80°C overnight, and transfer to liquid nitrogen for long-term storage the next day.
[0060] 2. Recovery of cryopreserved cells
[0061] After 15 days of cryopreservation, the above-mentioned frozen cells were resuscitated. Remove the cells from liquid nitrogen and quickly thaw in a 37°C water bath. The thawed cell suspension was centrifuged at 1000 rpm to collect th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com