FpAT freeze-dried preparation for injection and freeze-drying method thereof
A technology of angiogenesis and freeze-dried preparations, which is applied in freeze-dried delivery, cardiovascular system diseases, medical preparations of non-active ingredients, etc. It can solve the problems of preparation surface atrophy, excessive water content, drug inactivation, etc. Effects of biological activity, reducing pH change, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This embodiment provides a preparation method of angiostatin freeze-dried preparation for injection, comprising:
[0061] formula:
[0062]
[0063] Specific steps are as follows:
[0064] Firstly, prepare the solution, add 1 g of the angiogenesis inhibitory peptide raw material to 50 mL of water for injection to make a solution, filter it with a 0.22 μm membrane aseptically for use, and obtain the polypeptide solution;
[0065] Weigh 16 g of auxiliary materials dextran and 2.56 g of sodium hydroxide and add them into 50 mL of water for injection to make a solution, filter through a 0.22 μm membrane to make a pyrogen-free sterile auxiliary material aqueous solution, and cool;
[0066] Mix the peptide solution and the excipient solution, add water for injection to 200mL, and the pH is 9.5; put the mixed solution into a clean and sterile vial, stop it halfway with a rubber stopper, a total of 100 vials, and freeze-dry;
[0067] Wherein, the freeze-drying process incl...
Embodiment 2
[0078] This embodiment provides a preparation method of angiostatin freeze-dried preparation for injection, comprising:
[0079] formula:
[0080]
[0081] Specific steps are as follows:
[0082] Firstly, prepare the solution, add 10 g of the angiogenesis inhibitory peptide raw material to 500 mL of water for injection to make a solution, filter it with a 0.22 μm membrane for sterile filtration, and obtain the polypeptide solution;
[0083] Weigh 40 g of excipients mannitol and 6.24 g of sodium dihydrogen phosphate and add them into 1000 mL of water for injection to form a solution, filter through a 0.22 μm membrane to make a pyrogen-free sterile excipient aqueous solution, and cool;
[0084] Weigh 5 g of solid sodium hydroxide and dissolve it in 100 mL of water for injection, and filter it with a 0.22 μm membrane for use to obtain an alkaline solution;
[0085] Mix the peptide solution and the excipient solution, adjust the pH to 7 with alkaline solution; add water for i...
Embodiment 3
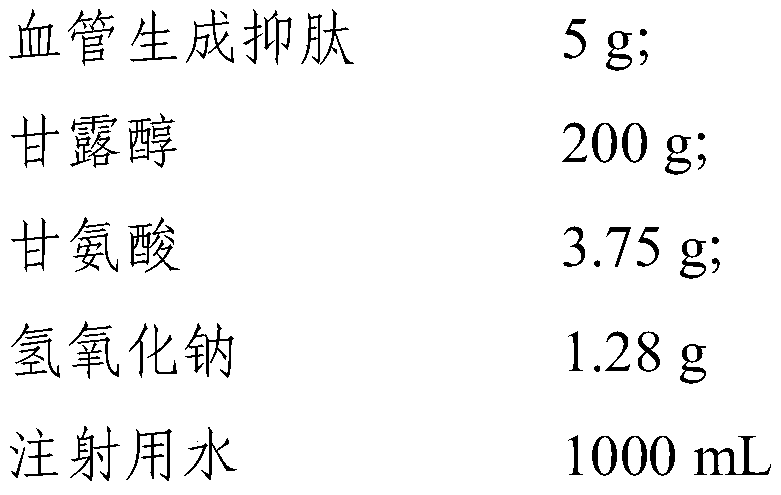
[0100] This embodiment provides a preparation method of angiostatin freeze-dried preparation for injection, comprising:
[0101] formula:
[0102]
[0103] Specific steps are as follows:
[0104] Firstly, the solution was prepared by adding 5 g of the angiogenesis inhibitory peptide raw material into 300 mL of water for injection to make a solution, and sterile-filtered it with a 0.22 μm membrane for use to obtain a polypeptide solution;
[0105] Weigh 200 g of excipients mannitol and 3.75 g of glycine and add them into 500 mL of water for injection to make a solution, filter through a 0.22 μm membrane to make a pyrogen-free sterile excipient aqueous solution, and cool;
[0106] Weigh 1.28 g of solid sodium hydroxide and dissolve it in 100 mL of water for injection, filter it with a 0.22 μm membrane aseptically to obtain an alkaline solution;
[0107] Mix the polypeptide solution and the excipient solution, adjust the pH to 9 with alkaline solution; add water for injectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com