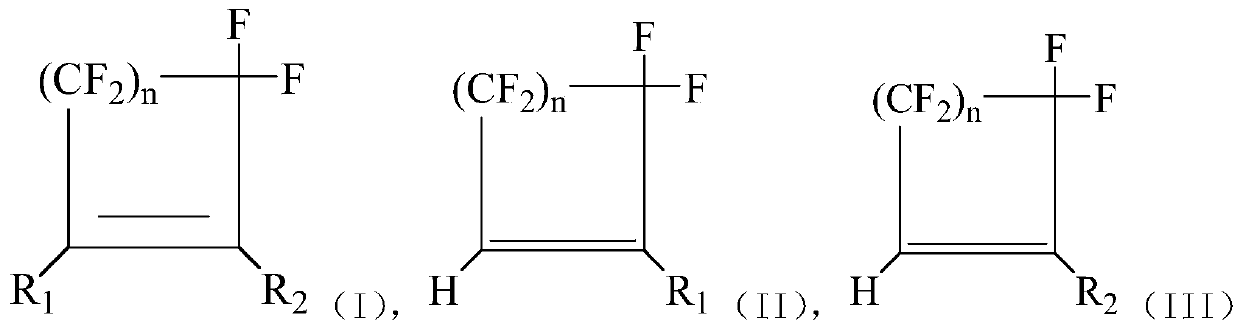
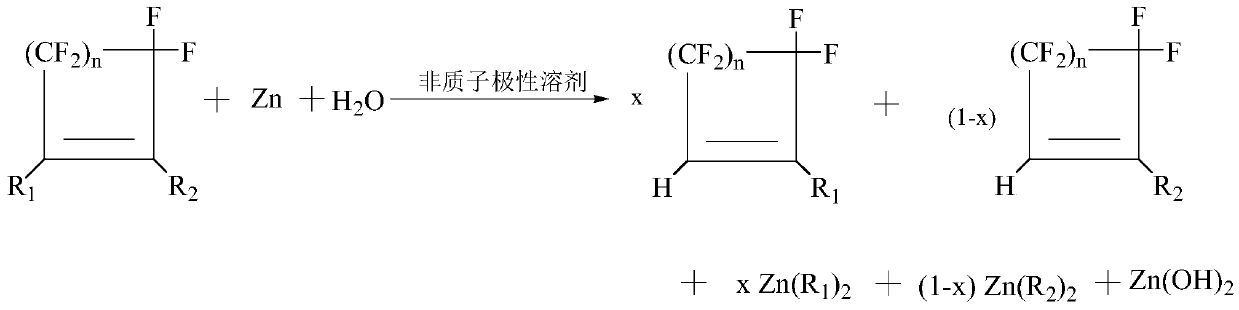
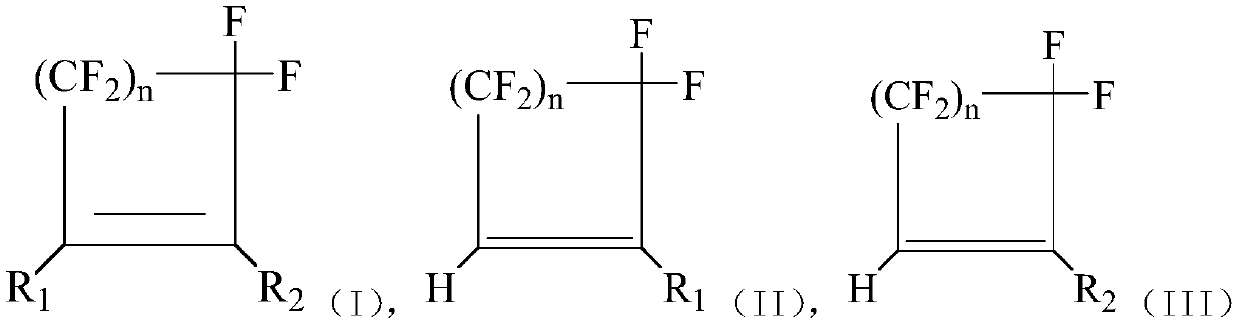
Method for preparing hydrogen halide cycloolefin by hydrolyzing halogenated cycloolefin
A technology for hydrohalogenated cyclic olefins and halogenated cyclic olefins, which is used in dehalogenation preparation, organic chemistry, etc., can solve the problems of high reaction temperature, low selectivity, and pollute the environment, and achieves safe and reliable process, mild reaction conditions, and post-processing Handling easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add zinc powder, water, N,N-dimethylacetamide, and 1,2-dichlorohexafluorocyclopentene to a 200-mL glass flask equipped with a condenser and a balloon-sealed condenser outlet under stirring conditions , the molar ratio of zinc powder / water / N,N-dimethylacetamide / 1,2-dichlorohexafluorocyclopentene is 1.25 / 1 / 5 / 1, zinc powder is 0.25 moles, water is 0.2 moles, N , N-dimethylacetamide is 1 mole, 1,2-dichlorohexafluorocyclopentene is 0.2 mole, the reaction temperature is 80° C., and the reaction time is 6 hours. After reaction finishes, cool to room temperature, wash with 200 milliliters of water, filter to remove solid, filtrate is carried out normal pressure distillation, obtains 1-chloro-3,3,4,4,5,5-hexafluorocyclopentene (boiling point is 72-73°C / 760mmHg), the yield is 97.5%, and the purity is 99.6%.
Embodiment 2
[0042] Add zinc powder, water, N,N-dimethylacetamide, and 1,2-dichlorohexafluorocyclopentene to a 200-mL glass flask equipped with a condenser and a balloon-sealed condenser outlet under stirring conditions , the molar ratio of zinc powder / water / N,N-dimethylacetamide / 1,2-dichlorohexafluorocyclopentene is 1 / 1 / 5 / 1, zinc powder is 0.2 moles, water is 0.2 moles, N , N-dimethylacetamide is 1 mole, 1,2-dichlorohexafluorocyclopentene is 0.2 mole, the reaction temperature is 80° C., and the reaction time is 6 hours. After reaction finishes, cool to room temperature, wash with 200 milliliters of water, filter to remove solid, filtrate is carried out normal pressure distillation, obtains 1-chloro-3,3,4,4,5,5-hexafluorocyclopentene (boiling point is 72-73°C / 760mmHg), the yield is 88.0%, and the purity is 99.8%.
Embodiment 3
[0044] Add zinc powder, water, N,N-dimethylacetamide, and 1,2-dichlorohexafluorocyclopentene to a 200-mL glass flask equipped with a condenser and a balloon-sealed condenser outlet under stirring conditions , the molar ratio of zinc powder / water / N,N-dimethylacetamide / 1,2-dichlorohexafluorocyclopentene is 1 / 2.78 / 4.86 / 1, zinc powder is 0.2 moles, water is 0.556 moles, N , N-dimethylacetamide is 0.972 mol, 1,2-dichlorohexafluorocyclopentene is 0.2 mol, the reaction temperature is 80° C., and the reaction time is 6 hours. After reaction finishes, cool to room temperature, wash with 200 milliliters of water, filter to remove solid, filtrate is carried out normal pressure distillation, obtains 1-chloro-3,3,4,4,5,5-hexafluorocyclopentene (boiling point is 72-73°C / 760mmHg), the yield is 86.5%, and the purity is 99.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com