Preparation method of clotrimazole
A technology of clotrimazole and chlorophenyl, which is applied in the field of medicinal chemistry, can solve the problems of inconsistency, unfavorable industrial production, high equipment and operation requirements, etc., to reduce the risk of impact, shorten the process cycle, and reduce the cost of equipment and operation required effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
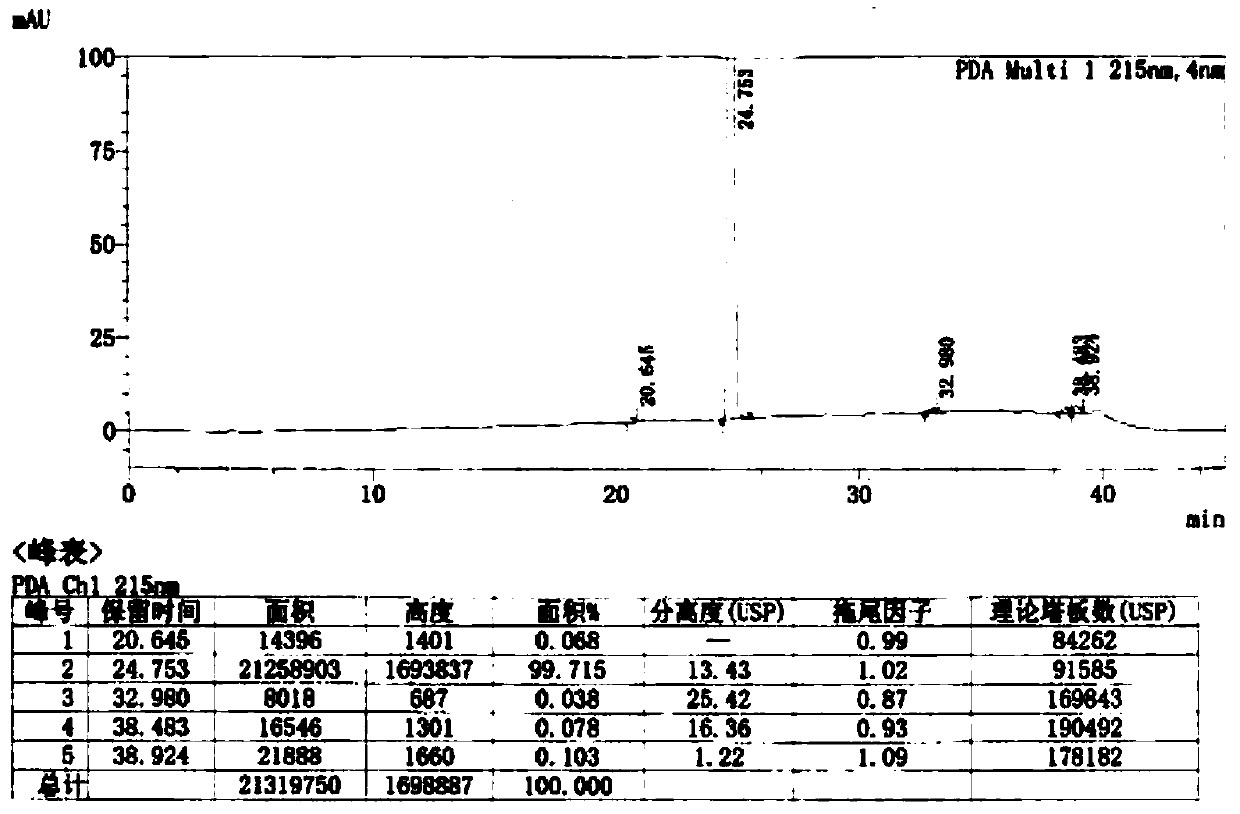
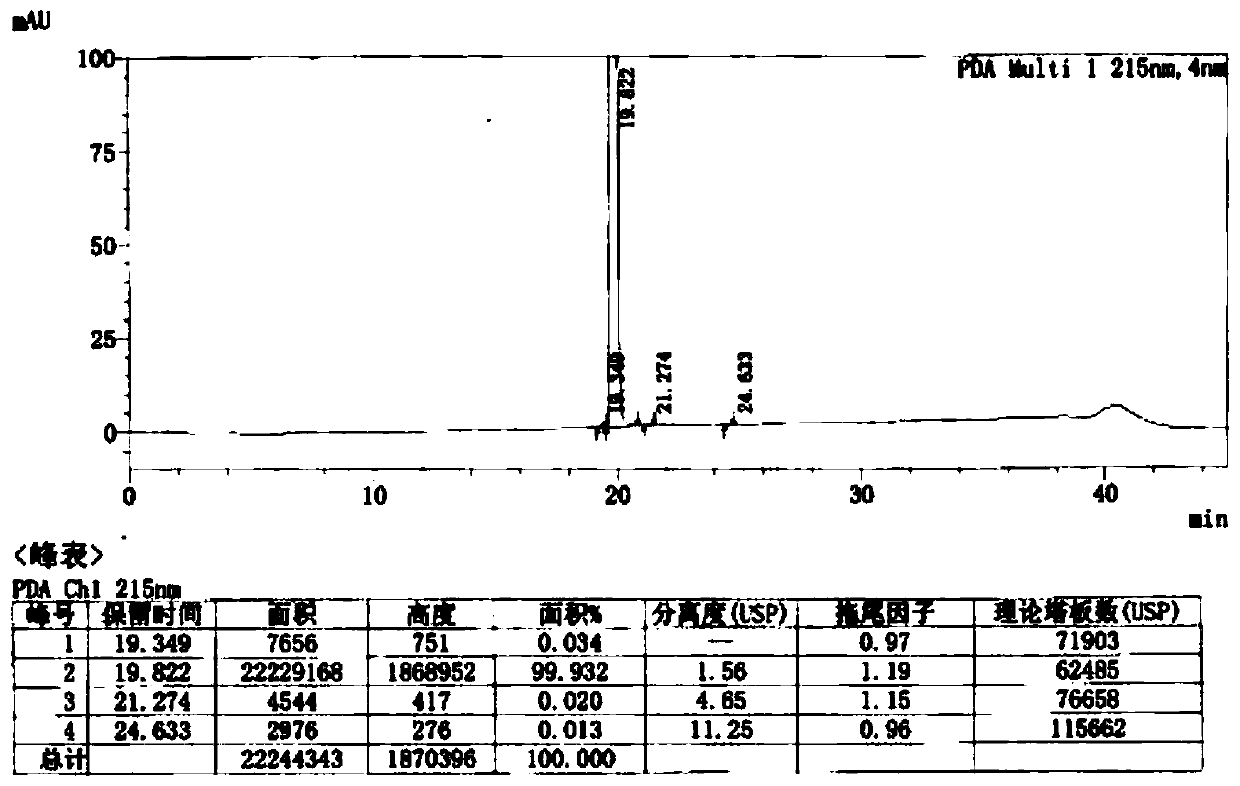
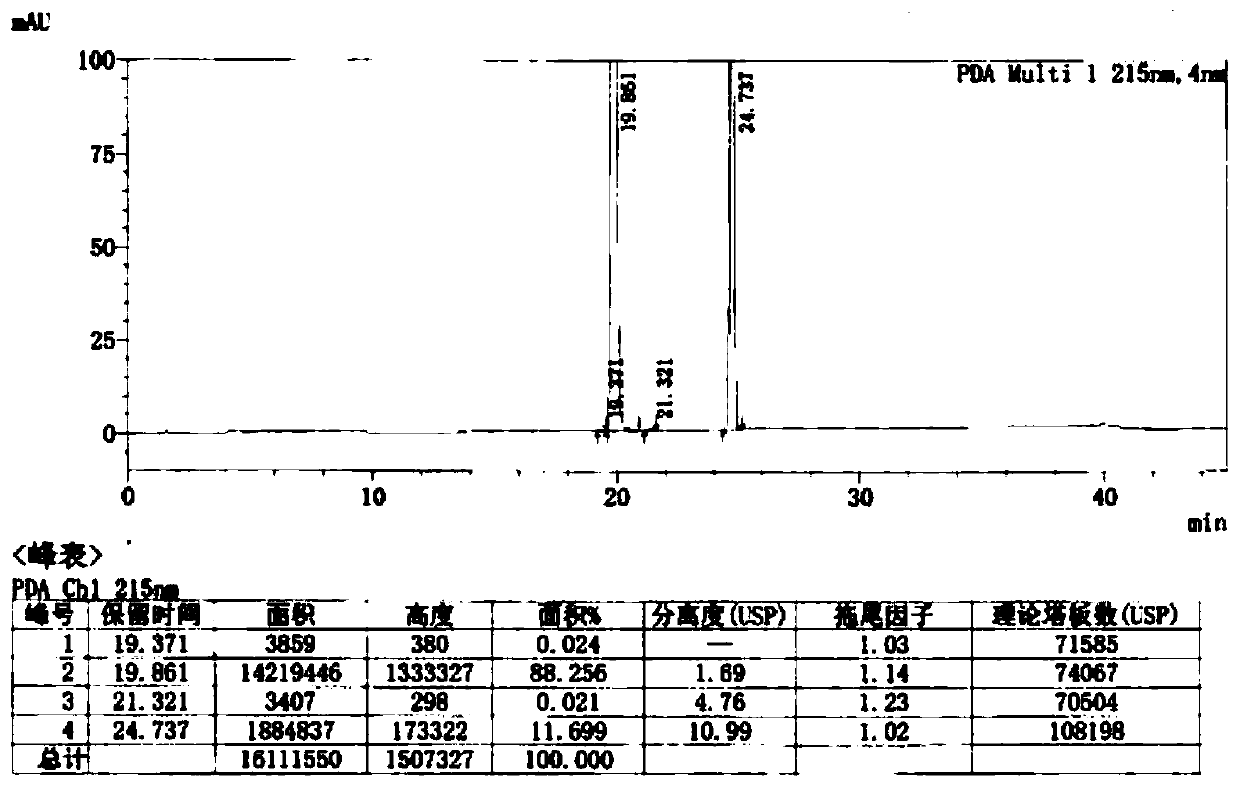
Image
Examples
Embodiment 1
[0028] This embodiment discloses a preparation method of clotrimazole, including step 1 and step 2, the overall reaction flow formula is:
[0029]
[0030] Step 1: (2-chlorophenyl) diphenylmethanol is chlorinated into (2-chlorophenyl) diphenylchloromethane, and the specific operations are as follows:
[0031] Add 1L of toluene and 100g of (2-chlorophenyl) benzylmethanol to a 2L three-necked flask, stir to dissolve, add 5ml of N,N-dimethylformamide and 10g of thionyl chloride, and raise the temperature to 60-65°C to initiate , start to add 91g of thionyl chloride dropwise after triggering, and control the temperature during the process not to exceed 65°C.
[0032] After the reaction was completed, the temperature was raised to 90-95°C, and atmospheric distillation was started, and the distillation was continued for 2 hours after no obvious fractions flowed out. The temperature was lowered to 30-35°C, and distillation under reduced pressure was carried out at this temperatur...
Embodiment 2
[0037] This embodiment discloses a preparation method of clotrimazole, including step 1 and step 2, the overall reaction flow formula is:
[0038]
[0039] Step 1: (2-chlorophenyl) diphenylmethanol is chlorinated into (2-chlorophenyl) diphenylchloromethane, and the specific operations are as follows:
[0040] Add 500L of toluene and 100g of (2-chlorophenyl) benzylmethanol to a 1L three-necked flask, stir to dissolve, add 5ml of N,N-dimethylformamide and 10g of thionyl chloride, and raise the temperature to 60-65°C to trigger. After initiation, 91 g of thionyl chloride was added dropwise (the temperature was controlled not to exceed 65° C. during the process). After the dropwise addition, keep warm and stir at 60-65°C for 2h.
[0041] After the reaction is completed, cool the reaction solution to 30-40°C, concentrate under reduced pressure at 30-40°C, add 500mL of toluene after solid precipitation, continue to concentrate under reduced pressure at 30-40°C, and collect about...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com