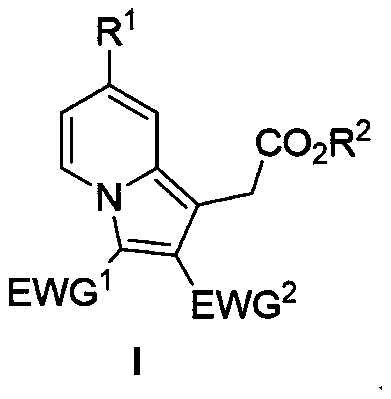
Polysubstituted indazine derivative and preparation method thereof
A multi-substitution and derivative technology, applied in the direction of organic chemistry, can solve the problems of cumbersome operation, and achieve the effects of simple operation, easy mass preparation, and no metal pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
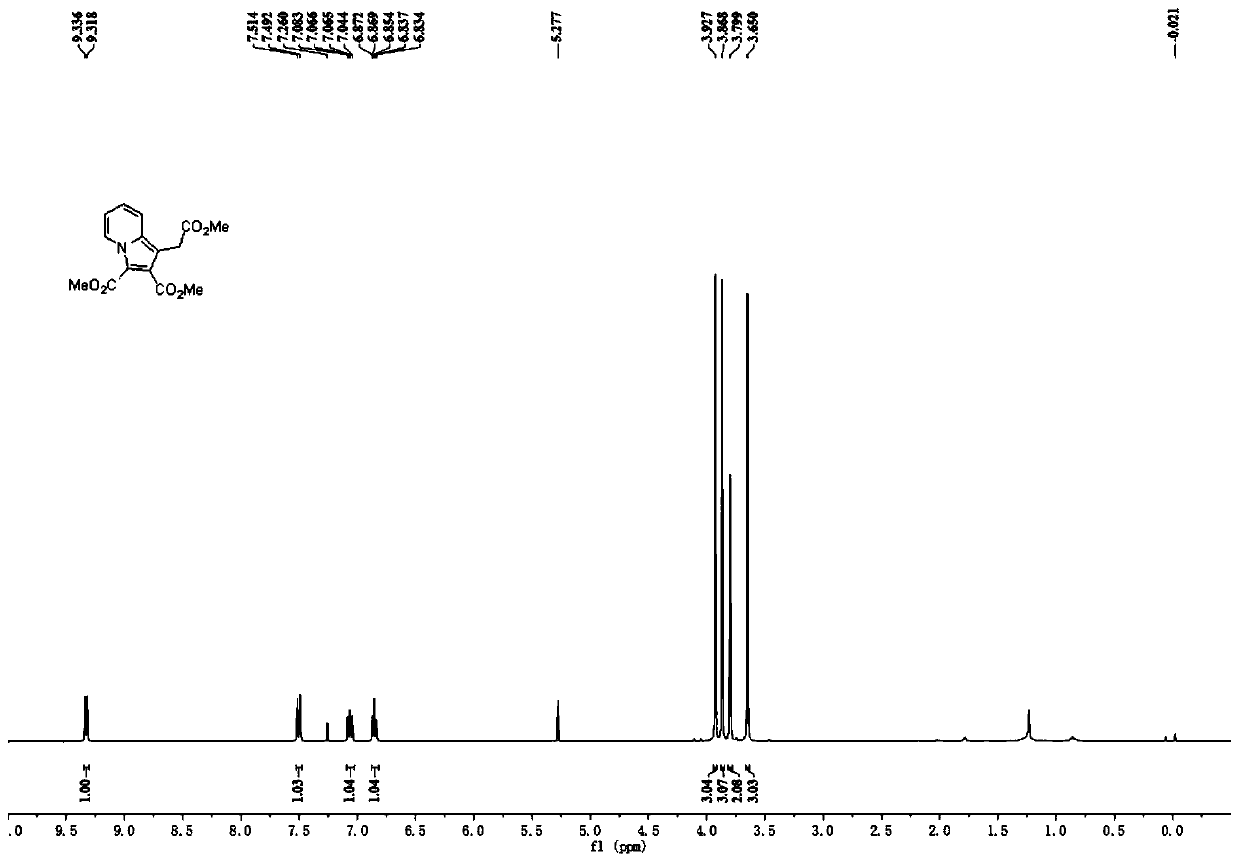
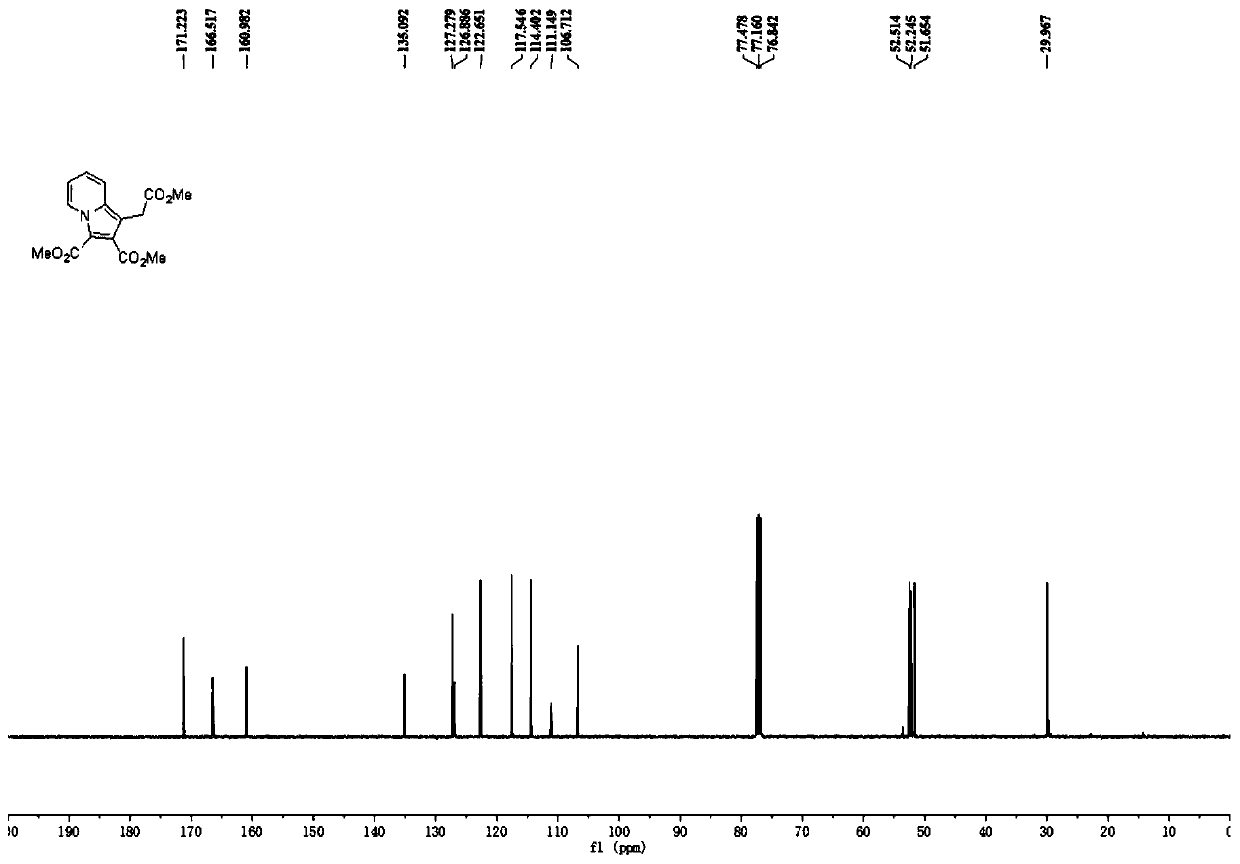
[0023] The reaction formula of Example 1, the specifically used compound III-1 and compound II-1 and the structure of the product I-1 are shown in the following formula. Experiment shows that the present invention uses salt of wormwood and triethylamine as mixed base, and preferred organic solvent is methylene dichloride, and the highest yield of its reaction product is 68%, and the best raw material molar ratio is compound III: compound II: potassium carbonate : Triethylamine=1.0:4.0:2.0:2.0, wherein compound III is an equivalent value, and compound II needs to be in excess to ensure that compound III can react completely, and the optimal concentration of the solution is 0.1M.
[0024]
[0025] The specific experimental steps are: dissolve 127mg (0.50mmol, 1.0 equivalent) of compound III-1 and 168mg (2.0mmol, 4.0 equivalent) of compound II-1 in 5mL of dichloromethane, add 138mg (1.0mmol, 2.0 equivalent) ) of potassium carbonate, 101mg (1.0mmol, 2.0 equivalents) of triethyl...
Embodiment 2
[0029] The specific experimental steps for the preparation of compound I-2 to compound I-11 are the same as those for the preparation of compound I-1, and the structures of the raw materials used are as follows:
[0030]
[0031] The resulting product structure and data are characterized as follows:
[0032]
[0033] Product Ⅰ-2 is a yellow solid with a yield of 64%; melting point: 46-47°C. 1 H NMR (300MHz, CDCl 3)δ9.32(d, J=7.2Hz, 1H), 7.51(d, J=9.2Hz, 1H), 7.04(ddd, J=9.2Hz, 6.8Hz, 1.2Hz, 1H), 6.84(td, J =7.0,1.6Hz,1H),4.10(q,J=7.2Hz,2H),3.92(s,3H),3.86(s,3H),3.78(s,2H),1.20(t,J=6.8Hz ,3H); 13 C NMR (75MHz, CDCl 3 )δ169.9, 165.5, 160.0, 134.2, 126.1, 126.0, 121.6, 116.7, 113.5, 110.4, 106.4, 60.2, 51.5, 50.8, 29.3, 13.5; ESI-HRMS m / z calcd for C 16 h 18 NO 6 [M+H] + 320.1129,found 320.1123.
[0034] Product Ⅰ-3 was a yellow oily liquid with a yield of 60%. 1 H NMR (400MHz, CDCl 3 )δ9.33(d, J=6.8Hz, 1H), 7.53(d, J=8.8Hz, 1H), 7.06(t, J=7.4Hz, 1H), 6.84(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com