A-D-A type indacenodithiophene nuclear terminal methyl micromolecule and preparation method thereof
A technology of A-D-A and dithiophene, applied in the field of organic solar cells, can solve the problems of weak absorption, limited development, difficult adjustment of energy level and absorption spectrum, etc., and achieve the effect of less pollution and less energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below in conjunction with the accompanying drawings.
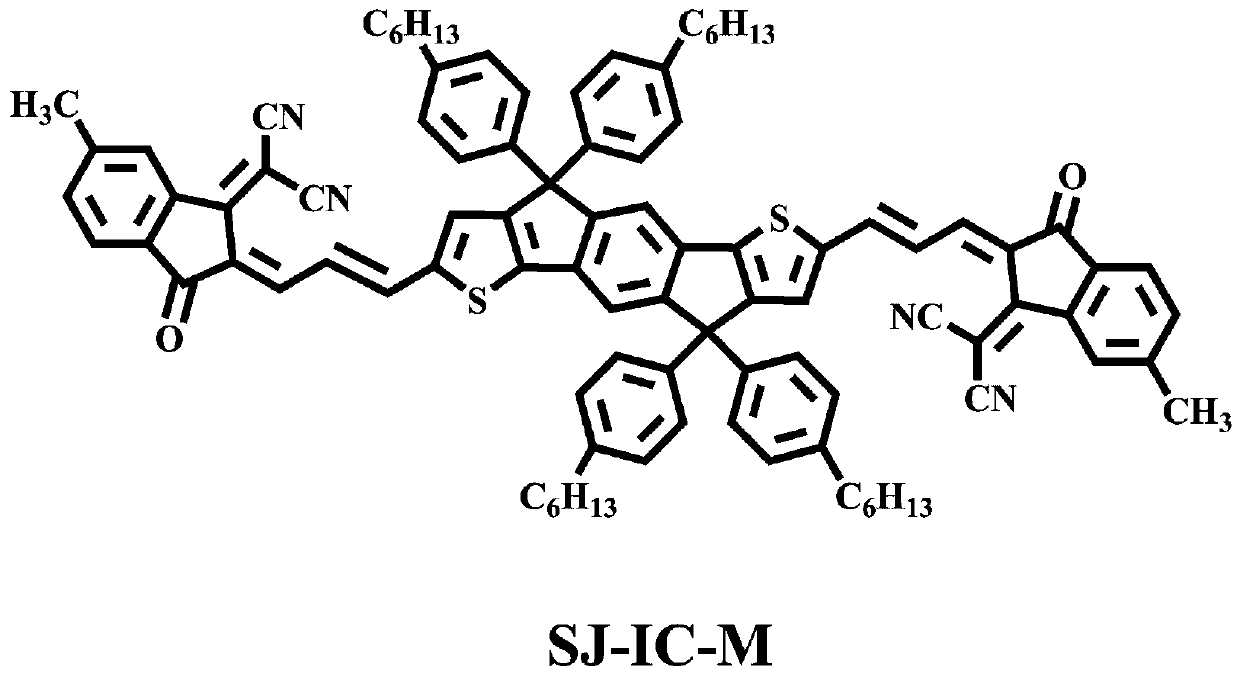
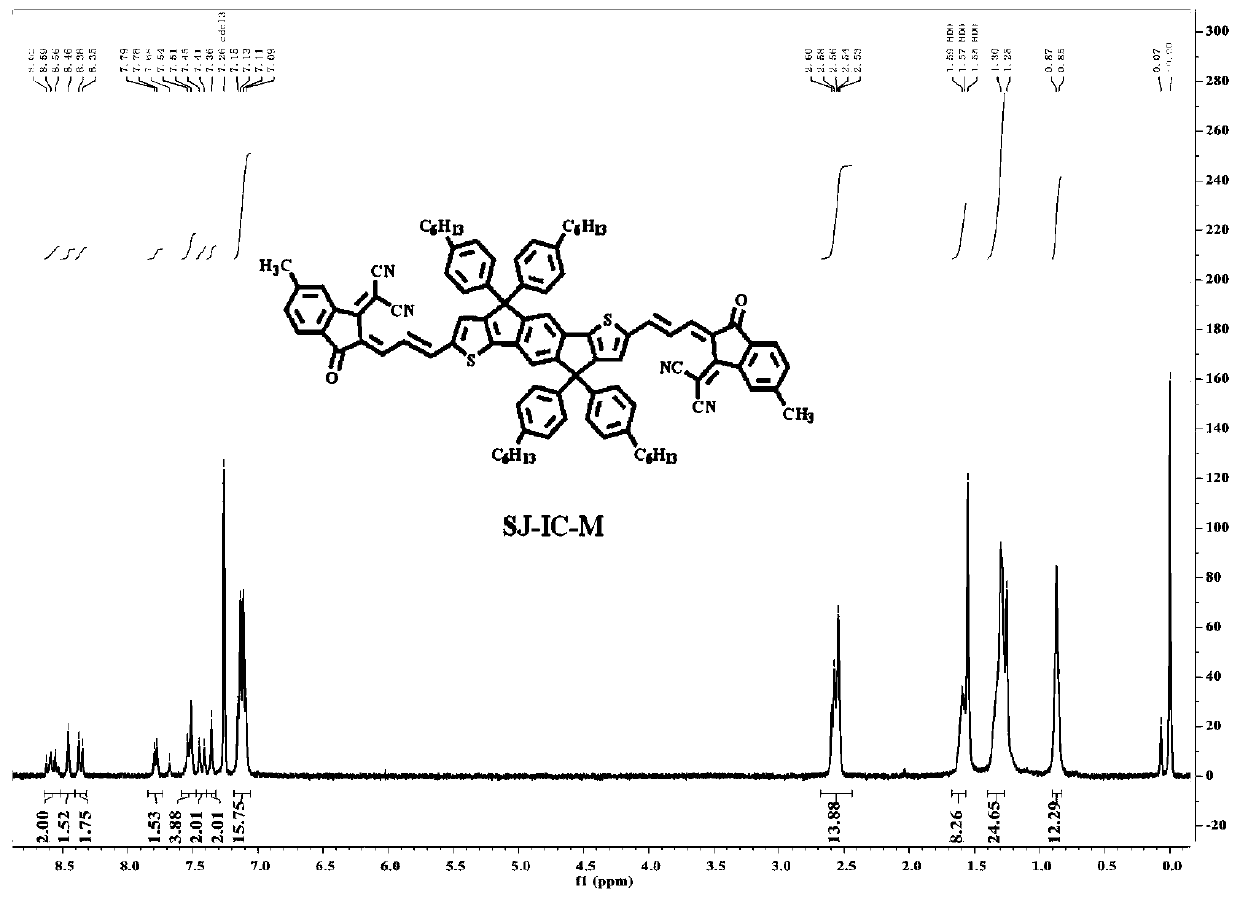
[0031] The reaction equation of the present invention is attached Figure 4 , the specific reaction steps are as follows: a small molecule of A-D-A type indanodithiophene nucleoterminal methyl group synthesis steps:
[0032] (1) Synthesis of SJ-CHO: Add 0.5mmol IDT-CHO and 0.6mmol tributyl(1,3-dioxazolidine-2-methyl)phosphine bromide to a dry 100mL nitrogen bottle, and add 20mL of anhydrous tetrahydrofuran, magnetically stirred for 5min to fully dissolve. Add 1.5 mmol of sodium hydride into the above-mentioned reaction nitrogen bottle under the condition of ice bath, remove the ice bath after adding and raise to room temperature, and react at room temperature for 16 h. After the reaction was completed, excess NaH was quenched with a cooled 10% HCl solution, the reaction mixture was adjusted to acidic pH, and stirred at room temperature for 4-5 h. The contents of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com